Abstract
Purpose
To use impression cytology to examine the structural changes in corneal epithelial cells infected with the herpes simplex virus in rabbit eyes.
Methods
Corneal surfaces of 7 rabbits were scratched using a 25-gauge needle. Herpes simplex virus (type 1, Kos strain) was inoculated to the injured cornea. As the corneal diseases were observed using slit lamp biomicroscopy, impression cytology was performed for 18 days after inoculation. Specimens were stained with hematoxylin-eosin and examined using optical microscopy.
Results
Corneal lesions consisted mainly of round epithelial cells, inflammatory cells, ballooning cells, multinucleated giant cells, and various inclusion bodies. Over time, the corneal epithelial cells peeled away as a result of corneal edema in the corneal lesions. Dendritic lesions were also observed. In the recovery phase, the number of detached cells and infiltrated inflammatory cells decreased.
The herpes simplex keratitis has been one of the main causes of blindness due to keratitis. The herpes simplex virus (type 1, Kos strain) develops into various types of keratitis and dendritic or geographic keratitis is the distinctive clinical finding.1-3 This type of epithelial keratitis is caused by damage generated from the actively proliferating virus. Unsuccessful treatment of herpes simplex epithelial keratitis or repetitive recurrence of keratitis may accompany stromal keratitis, and leads further to corneal opacity and visual deterioration, which is considered as the most commonly observed clinical impression.1,2
Other than scanning electron microscopy, the authors noticed that there have not been any clinical studies regarding the morphological changes of the epithelial surfaces relative to the elapsed time of the epithelial keratitis which developed either from the herpes simplex virus or from other disorders which could allow herpes simplex keratitis to induce visual disabilities.4 The authors therefore induced regional damage to the corneal epithelial cells in rabbit eyes and infected this area with the herpes simplex virus in order to experimentally develop herpes simplex keratitis and facilitate examination with impression cytology of the structural changes in the corneal epithelial cells over time.
The Vero cells were placed into the culture media embedded with modified essential media (Difco, U.K) which was composed of 5% fetal calf serum. The monolayered Vero cells were administered to grow in the incubator. The suspension of the frozen stored herpes simplex virus (Type 1, Kos Strain) was administrated to the culture dishes where the monolayered Vero cells were grown. When the Vero cells showed cytopathic effect, the cellular cultivation was interrupted and the virus suspension was prepared with filter paper.
Seven rabbits were used as the test subjects. After the rabbit eyes were instilled with 0.5% proparacaine, the focal section of the corneas were scratched several times horizontally and vertically with a 25-gauge needle to damage the corneal epithelium and then the suspension of herpes simplex virus type-1 (Kos strain) was dropped onto the damaged area. After inoculating the corneas with the virus, all eye drops to treat the corneal lesions were prohibited. The corneal lesions were observed daily using slit lamp biomicroscopy until the 18th day of the virus inoculation and, impression cytology was performed whenever any corneal lesions developed.
After dropping the cornea with 0.5% proparacaine, filter paper (Millipore filter 7×7 mm, 0.22 um, GS type, Ireland) was placed on the corneal lesion which was compressed lightly with the plunger tip of the 1ml tuberculin syringe. After 20 seconds, the filter paper was removed from the cornea and fixed with 10% formalin solution. Then the filter paper was stained with hematoxylin-eosin, but not PAS, because the filter paper stain was for the corneal surface.
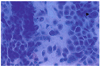
By day 3 of the virus inoculation, the polymorphonuclear leukocyte and the lymphocyte were infiltrated as the epithelium cells were desquamated away from the superficial layer of the corneal epithelium (Fig. 1). Balloon cells featuring plentiful cytoplasms and faint signs of a nucleus were observed in the lesion where the corneal epithelial cells were desquamated away and the fibrin deposits were displayed nearby (Fig. 2). Whereas the epithelial lesions showing dendritic keratitis were desquamated relatively deeper than the other areas, the epithelium cells in the nearby areas did not exhibit intensive desquamation.
By day 5, the corneal lesions initially observed on the surface were widened, the corneal epithelium was further desquamated off, and the changes in corneal epithelial cells were more intensified. Moreover, increased formation of balloon cells and irregular figures of superficial epithelial cells and wing cells were observed (Fig. 3). Infiltrations of the inflammatory cells such as polymorphonuclear leukocytes had increased as well.
By day 7, desquamations of the corneal epithelium were continuously proceeding and thus snake cells, appearing like chromatin clumping and multinucleated giant cells, were observed. The number of inflammatory cells infiltrated into the gaps between the cells was increased. When observed under high magnification, the inflammatory cells appeared lighter than the epithelial cells and the surrounding tissues were observed to be necrotic tissues and membranous materials which constituted of the transformed inflammatory materials. Plentiful cytoplasm and vacuoles were observed within the balloon cells and uniformed inclusion bodies of high density were observed within the cell nuclei (Fig. 4).
By day 11, the wing, snake and multinucleated giant cells had all become more numerous and the peripheral basal cells featured plentiful cytoplasm and ultrabasic transformed nuclei. Moreover, the intranuclear inclusion was detected and the fibrin was more prominently deposited to the surroundings (Fig. 5).
By day 13, the scratched area was observed with the presence of edema and desquamated epithelial cells. However, whereas the desquamated corneal epithelial cells and wing cells were relatively uniform and consistent, the multinucleated giant cells and the intranuclear inclusions were still increasing (Fig. 6).
By day 15, multinucleated giant cells and intranuclear inclusions were observed. However, the corneal epithelial cells demonstrated a relatively uniform shape and the number of inflammatory cells started to decrease (Fig. 7).
By day 17, the number of desquamated cells started to decrease and the infiltration of inflammatory cells had almost disappeared (Fig. 8).
Impression cytology was first introduced by Egbert in 1977 and the main advantages of this technique were safety, simplicity and repeated applicability. This technique has been applied to enable prompt diagnosis of dry eye syndrome, allergic keratoconjunctivitis and epithelial lesions of the eyes such as inflammation of the cornea and conjuntiva.5 Since the cornea sample of impression cytology can accurately reproduce the dendrite lesion down to a diameter of 0.3 mm in corneal epithelial disease, impression cytology has further uses for immunocytologic examination and the diagnosis of bacterial keratoconjuntivitis.6-9
Herpes simplex keratitis is the form of keratitis developed from the herpes simplex virus and displays various forms of inflammatory reactions. The changes in the initial stage can be similar to that of keratoconjunctivitis which developed from other viruses. However, the unique changes in the epithelial dendrite in the initial phase allow it to be distinguished from other corneal diseases.10 Even though are various clinical features and different perspectives regarding the cause of the disease, dendritic epithelial keratitis has been regarded as the most characteristic impression in almost all findings.
The experiment which introduced the herpes simplex virus (Kos strain) into a rabbit resulted in clinical phases that were similar to that from the application of intrastromal injection, scratching method, and epithelial abrasion method.11 Currently, the cytopathologic effect of keratitis by this herpes simplex virus has been relatively well-recognized. However, there have been no observations regarding the changes in corneal epithelial cells by the herpes simplex virus under light microscopy.
In this experiment, the changes in the corneal epithelial cells were observed for 18 days after the corneal epithelial cells were damaged and inoculated with type 1 herpes simplex virus (Kos strain). During this period, the observation of the epithelial impression in herpes simplex keratitis showed that the epithelial cells were desquamated, that the wing cells or basal cells started to become exposed from the superficial layer of the corneal epithelial cells, and that the inflammatory cells were infiltrated into the epithelium through the lesion where the epithelial cells were desquamated. These findings indicated that the epithelium cells infected with the herpes simplex virus had expanded the gap between the epithelial cells of the cornea and that this then led to the disintegration of the adhesion complex and the desquamation of the epithelium from the epithelial cells of the superficial layer.4,12 The expansion of the gap between the cells and the cellular desquamation induced the inflammatory cells which originated from the tear or limbus to infiltrate into the corneal epithelial cells. The change in the epithelial cells was further catalyzed by the cytokines isolated from the inflammatory cells.12
In this study, the infected epithelium cells were observed with desquamated and transformed corneal epithelial cells, which exhibited a fattened or minor transformed nucleus, and with multinucleated giant cells, which exhibited an irregular patterned nucleus. Possible evidence of virus-infected cells such as intranuclear inclusion was observed and this impression was similar to that exhibited from the nucleus of the stromal cells in the case when both the corneal stromal cells and herpes simplex virus were cultivated simultaneously.11-15 Moreover, the appearance of snake cells could be regarded as indicating the transformation of the surrounding cells by the infection of the herpes simplex virus and the inflammatory mediators and toxic material of the necrotic cells. Knop et al. noted that these cells contribute to the causes of the nucleus deformation through the physical stimulus and the transformation of the cytoskeleton.16 The small and round epithelial cells which composed ballooned nucleus were recognized as balloon cells, which had therefore originated from the corneal epithelial cells. By day 7, during the active stage, inflammatory cells and basal epithelial cells were observed and these were boldly surrounded by either a superficial layer arranged in 3 to 4 rows or the intermediate corneal epithelial cells. By day 15, during the recovery period, either the abundant superficial layer or the intermediate corneal epithelial cells and the rare basal epithelial cells and inflammatory cells were observed. Unlike the superficial layer or the intermediate corneal epithelial cells, the basal epithelial cells were composed of a strong basophilic nucleus and scanty cytoplasm. They were mainly observed in the middle of the corneal lesions whereas the superficial layer or the intermediate corneal epithelial cells were mainly observed in the surrounding areas of the corneal lesions. This may suggest that the infected cells underwent further specialization into multinucleated giant cells or that the intracellular inclusion originated from the basal epithelial cells from the corneal dendritic lesion. Moreover, these deformations in the nucleus and cytoplasm of the basal epithelial cells may have been stimulated by the virus-induced cellular activities.17
In this experiment, the corneal dendritic lesion became observable from day 3 of the inoculation but had disappeared by day 14 or 15. There was no significant difference among the rabbits in terms of lesion forms, nor in the lesion appearance and disappearance days in terms of the inoculation method. It was thought that the relative consistency of the results with respect to the inoculation method may have been due to the specific characteristic of the Kos strain used for the experiment. Kim et al. also reported similar results for rabbits by day 2 of the inoculation after applying the epithelial abrasion method.11 In the study of epithelial keratitis in rabbits infected with the same herpes simplex virus of Kos strain by Kim et al, dendritic and punctate lesions were reported to appear from day 1 of inoculation and the maximum appearance of dendritic lesions was observed by day 8; however, these types of lesions had completely disappeared by day 14.11 Vrabec and Darrell examined the temporal and histological findings exhibited from corneal lesions which appeared after the rabbit had been inoculated with the virus.18 A lesion, which was only identified histologically began to appear within 7 hours from the inoculation and a dendritic lesion appeared in slit lamp examination within 24 hours. Furthermore, the lesion was consistent for a period of 2 weeks or 25 days with respect to the virus strain. The dendritic lesion was observed in the adjacent area of epithelial abrasion and it was speculated that this area could have been infected by the virus directly.
The pathogenesis of the dendritic ulcer induced by the herpes simplex virus was not completely verified. However, it was considered that the lesion might have appeared with respect to the nerve distribution on the epithelial layer or that the segmentation might have occurred in accordance with the differences in individual cellular resistance against the infection. Kim et al. indicated that in the case of the sample being inoculated by the scratching method, the discontinuous lesions were generated within a certain distance of the scratch, and that in the case of the lesion proceeding through dendrites, it was proceeded by segmentation after considering this generation region as the center, and that this suggested that the possible existence of the virus growth resistance factor was secreted from the epithelial cells in prohibiting the extensive process of lesion development.11 However, the herpes simplex virus was either clinically or experimentally indicated as the neurophillic virus, such that, it further demonstrated numerous evidences of viral development with respect to the nerve distributions, such as the deterioration of corneal sensitivity, latency and recurrence in clinical examination, and so the possible development of lesions with respect to the nerve distributions should be kept in mind.19
Conclusively, the infection of corneal epithelial cells by the herpes simplex virus induces cellular desquamation and inflammatory cellular infiltration. The infected epithelial cells might undergo changes in the nucleus such as intranuclear inclusion. The injury recovery process of the epithelial cell had commenced at approximately 2 weeks after the initial infection.. This experiment was beneficial in expanding our knowledge of the changes of epithelial keratitis with respect to elapsed time. However, since this experiment used the Type 1 herpes simplex virus (Kos strain), which is known to cause less destructive effects, the results of this study can not be extended to generalize cases caused by other species of herpes simplex virus or accompanied by recurrence and stromal keratitis. Furthermore, further research is required in this area.
Figures and Tables
Fig. 1
Many inflammatory cells and degenerated corneal epithelial cells have infiltrated the corneal lesion, 3 days following inoculation of the herpes simplex virus (Impression cytology, H-E stain ×100).
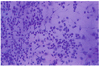
Fig. 2
Balloon cells (arrow) can be seen in the area of the detached and degenerated corneal epithelium, which had swollen cytoplasm and a pale nucleus, 3 days following inoculation of the herpes simplex virus (Impression cytology, H-E stain ×100).
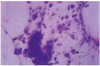
Fig. 3
Epithelial cells transferred from the lesion exhibited widening of the scratching wound lesion, 5 days following inoculation of the herpes simplex virus (Impression cytology, H-E stain ×100) (H-E stain ×40).
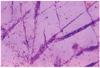
Fig. 4
Higher magnification of the dendritic keratitis lesion, 7 days following inoculation of the herpes simplex virus. There were an increased numbers of balloon cells (arrow), degenerated superficial cells with bizarre nucleus and vacuolated cells (arrowhead), 7 days following inoculation of the herpes simplex virus(Impression cytology, H-E stain ×400).

Fig. 5
The specimen obtained from the center of an actively infected lesion, showing syncytia (arrow) and epithelial cells including a dense basophilic nucleus. Snake cells (arrowhead) were observed, 11 days following inoculation of the herpes simplex virus (Impression cytology, H-E stain ×400).
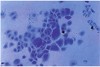
Fig. 6
Intranuclear inclusions (arrow) in a specimen of dendritic keratitis, 11 days following inoculation of the herpes simplex virus(Impression cytology, H-E stain ×400).
References
1. Hyndiuk RA, Glasser D. Tabbara KF, editor. Herpes simplex keratitis. Infections of the Eye. 1986. 1st ed. Boston: Little Brown;1204–1223.
2. Pepose JS, Leib DA. Stuart M, editor. Herpes simplex virus diseases. Ocular infection and immunity. 1996. v.1:1st ed. London: Mosby;chap. 71.
3. Dawson CR, Togni B. Herpes simplex eye infections: clinical manifestations, pathogenesis and management. Surv Ophthalmol. 1976. 21:121–135.
4. Lee KY, Chung MR, Ko MK. Scanning Electron Microscopic Observations of Sequential Alterations of Type 1 HSV Keratitis in Rabbits. J Korean Ophthalmol Soc. 2004. 45:1174–1180.
5. Egbert PR, Lauber S, Maurice DM. A simple conjunctival biopsy. Am J Ophthalmol. 1977. 84:798–801.
6. Arora I, Singhvi S. Impression debridement of corneal lesions. Ophthalmology. 1994. 101:1935–1941.
7. Maskin SL, Heitman KF, Lawton AW, Yee RW. Diagnostic impression cytology for external eye disease. Cornea. 1989. 8:270–279.
8. Nelson DJ. Impression cytology. Cornea. 1988. 7:71–85.
9. Simon MW, Miller DMT, Pflugefelder SC. Comparison of immunocytology to tissue culture for diagnosis of presumed herpesvirus dendritic epithelial keratitis. Ophthalmology. 1992. 99:1408–1420.
10. Tabery HM. Epithelial changes in early primary herpes simplex virus keratitis. Photomicrographic observations in a case of human infection. Acta Ophthalmol Scand. 2000. 78:706–709.
11. Kim DC, Ko MK. Slit - Lamp Examination of the Experimentally Induced HSV - 1 Keratitis. J Korean Ophthalmol Soc. 1998. 29:251–255.
12. Kwon JW, Han JH, Ko MK, Kim JW. Ultrastructure of Corneal Epithelial Cells Following Inoculation of Herpes Simplex Virus in Rabbit. J Korean Ophthalmol Soc. 2001. 42:349–354.
13. Wander AH, Centifanto YM, Kaufman HE. Strain specificity of clinical isolates of herpes simplex virus. Arch Ophthalmol. 1980. 98:1458–1461.
14. Metcalf JF, Michaelis BA. Herpetic keratitis in inbred mice. Invest Ophthalmol Vis Sci. 1984. 25:1222–1225.
15. Lee YJ, Ko MK, Kim JG. The Ultrastructure of Rabbit Keratocyte Infected by Herpes Simplex Virus. J Korean Ophthalmol Soc. 2000. 41:28–33.
16. Knop E, Reale E. Fine structure and significance of snakelike chromatin in conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1994. 35:711–719.
17. Maudgal PC, Missotten L. Histopathology of human superficial herpes simplex keratitis. Br J Ophthalmol. 1978. 62:46–52.
18. Vrabec F, Darrell RW. The corneal epithelium in experimental herpetic keratitis in rabbits. Doc Ophthalmol. 1970. 29:213–224.
19. McKee AP. The biology of herpes simplex virus. Invest Ophthalmol. 1963. 2:490–497.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download