Abstract
A 63-year-old female with candidemia following necrotizing pancreatitis developed clinical signs of chorioretinitis and underwent the systemic administration of voriconazole, after which anterior chamber inflammation and multiple, white, fluffy, chorioretinal lesions, under 1mm in diameter, were gradually resolved and visual acuity improved. We report the first Korean case of candida chorioretinitis successfully treated with the systemic administration of voriconazole.
Endogenous fungal infection of the eye has become more influential due to the difficulties of its diagnosis and treatment. Candida is normal flora present in respiratory, gastroenteric and female genital systems, and shows low virulence in healthy people. However, candida can cause severe diseases when the host defense is weakened and is the most common endogenous infection of the eye. Candida chorioretinitis reveals as single or multiple, fluffy, white, chorioretinal lesions, less than 1 mm in diameter, and accompanies retinal hemorrhage and overlying haze of vitreous inflammatory cells. In addition, this chorioretinitis commonly accompanies iridocyclitis, and the degrees of anterior chamber reaction are various, sometimes to cause hypopyon. Candida chorioretinitis is treated with the systemic administration of antimycotic, and, if necessary, intravitreal injection or vitrectomy may be required. However, none of the case reports has reported yet on its treatment with voriconazole, which is a new triazole antifungal agent.
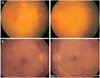
A 63-year-old female was admitted to the department of Internal Medicine on July 27, 2002, under the diagnosis of acute necrotizing pancreatitis, and was treated with antibiotics. This patient manifested fever from August 20, and showed candidemia on blood culture. The patient was tested in the department of Ophthalmology due to the complaints of conjunctival injection and ocular pain from September 1. The best corrected visual acuity (BCVA) was 0.2 in both eyes. On slit lamp exam, we observed tiny corneal precipitates and ++ of anterior chamber inflammation. Ocular pressure was 7 mmHg in both eyes. On fundus exam, we observed multiple, light yellowish, retinal infiltrations, mostly in the posterior pole, without overlying vitritis (Fig. 1A). The retinal infiltrations were in the deep retina. These deep retinal lesions, along with anterior chamber inflammation, favor the diagnosis of chorioretinitis rather than non-specific cotton wool spots or Purtscher's retinopathy, which are also seen in patients with pancreatitis. Under the diagnosis of acute necrotizing pancreatitis, candidemia and candida chorioretinitis, the patient intravenously received voriconazole from September 2. On the first day, 6 mg/kg of voriconazole was administered twice a day, after which the daily dosage was maintained at 3 mg/kg for 3 days, followed by oral administration (200 mg, twice a day) for 11 days. The patient complained of mild blurred vision, which resolved immediately without any other side effects. Homatropine was dropped into both eyes twice a day. On September 17, fever was resolved and the systemic conditions were improved on abdominal ultrasonography. Therefore, the patient was released from the hospital while maintained only on oral antibiotic without any voriconazole. The ophthalmological exam at the time of discharge found corrected vision of 0.4 in both eyes, traces of anterior chamber inflammation, ocular pressure of 10 mmHg in both eyes, and decreased fundus infiltration. In the follow-up examination on October 16, at 29 days post-discharge, in the outpatient clinic, we found improved visual acuity, anterior chamber inflammation and fundus finding. At this follow-up examination, BCVA was 0.7 in both eyes, the anterior chamber inflammation was resolved, the ocular pressure was 12 mmHg, and the infiltration on the fundus exam was almost gone (Fig. 1B).
In endogenous infectious endophthalmitis, the retinal lesion is rarely treated by solving the primary cause of fungemia without antifungal treatment. Furthermore, the failure of the treatment can induce high possibilities of severe endophthalmitis and its related vision loss. If the lesion is treated early, the prognosis of visual acuity is improved, and therefore, early diagnosis and antifungal treatment are necessary.1,2
The remedies are amphotericin B in the polyene class, flucytosine in the fluorinated pyrimidine class, and imidazole, ketoconazole, fluconazole and itraconazole in the azole class. Amphotericin B is a good agent against endogenous infectious endophthalmitis with its action to change the permeability of fungal cell membrane by irreversibly binding to ergosterol, the main sterol of the fungal cell membrane. However, is also regulated due to serious systemic side effects such as anaphylaxis, thrombocytopenia, flushing, generalized pain, convulsions, fever, chilling, phlebitis, headache, anemia, anorexia, etc. Moreover, intravitreous injection of amphotericin B has been considered as a possible means of treatment because of the poor intraocular penetration.3 Flucytosine inhibits the nucleic acid synthesis of fungi to be effective on fungal infection, and its single use has been reported as an effective remedy for candida chorioretinitis in a case report.4 However, the combination with other antimycotics is recommended because 50% of candidal organisms are resistant to flucytosine.5,6 Azole consists of imidazoles (miconazole, ketoconazole) and triazoles (fluconazole, itraconazole). Ketoconazole and miconazole have limited effects and poor penetration into the eyes. Itraconazole is more active than any other azole drug against Aspergillus species, but has poor intraocular penetration and little clinical experience. On the other hand, fluconazole shows excellent intraocular penetration so that its concentration in the retina and choroid is similar to that in the blood. It was reported that the efficacy of the single use of fluconazole was similar to the effects of the combined uses with amphotericin B and flucytosine. However, it has been suggested that fluconazole should be used as a secondary remedy after the primary use of amphotericin B, or when amphotericin B cannot be used. Furthermore, there are some species resistant to fluconazole. Voriconazole, a derivative of fluconazole, is a new triazole antifungal agent. Like other triazoles, this voriconazole inhibits cytochrome P450 demethylase essential for the synthesis of ergosterol, possibly be changing the permeability of the fungal cell membrane. This drug is active against various fungi including those resistant to fluconazole without significant side effects in the systemic administration, and maintains well its high concentration in blood and tissue. The representative side effects are light visual disturbance and liver function enzyme increase at the administration time, both of which are reversible. Other systemic side effects are the changes in the cardiovascular system, skin, gastroenteric system, renal function, etc. The reported visual disturbances are brightness, blurring, light sensitivity, and changes in color vision, etc., and approximately 30% of the patients experience these symptoms that start from 30 minutes after the administration and last for 30 minutes.
In reviewing a recent trend of fungal infection, the rates of strains resistant to the existing triazoles such as fluconazole and itraconazole have been increasing with the increasing rate of the infection by non-albicans candida and the infection by molds including Aspergillus.7 However, the infusion of amphotericin B can cause systemic side effects, and shows poor intraocular penetration. Moreover, there is no oral medication of amphotericin B. Therefore, several studies have progressed to the development of new antifungal agents, and voriconazole has proved to be effective for non-albicans candida resistant to fluconazole or itraconazole, and to have broad antifungal power against infection by Aspergillus or Scedosporium.7-9 In addition, voriconazole can enter the central nervous system to a greater extent than any other antimycotics, and therefore, can be more effective for fungal infection in the eyes and can be used in the outpatient clinics because the switch from injection to oral administration is possible owing to the excellent bioavailability (>90%) in the oral administration.8 Accordingly, the use of voriconazole as a primary drug is appropriate. A test result of the sensitiveness for the antimycotic, not performed in the present case, would be helpful in selecting the drug. The susceptibility testing against the fungus is not generally conducted on every patient because the established standard guidelines for the test of fungal sensitiveness are not ready, in contrast to those for bacteria, and the MIC values vary according to the conditions. In this case report, the patient did not exhibit any systemic side effect from the systemic administration of voriconazole, except for the complaint of light blurred vision which was resolved soon. Ophthalmological examination is necessary as a basic test prior to the administration of voriconazole, which can frequently be accompanied by the complaint of blurred vision. Moreover, the patient in this case complained of ocular pain and conjunctival injection, and underwent ophthalmological exam immediately to detect the patient's chorioretinitis. Subsequently, owing to the early diagnosis, this lesion was improved by the appropriate administration of the antimycotic without progressing into infectious endophthalmitis, and the prognosis for the vision was good. Therefore, the ophthalmological test for fungemia patients should be conducted even in the absence of any signs or symptoms. The importance of the ophthalmological test is greater in the prevalence of infectious endophthalmitis requiring other remedies, in addition to the administration of the antimycotic. Kim et al10 and Choi et al11 have reported on this importance.
Several reports were connected with in vitro, broad and effective reactions of voriconazole for the isolates of organs other than the eyes.12-16 However, only one report was related to the effects of voriconazole in comparison with other antimycotics for the isolates of the eyes.9 Furthermore, there is no case report on the treatment of candida chorioretinitis with only voriconazole administration. The present case is one of the clinical cases reporting that voriconazole has excellent effectiveness for chorioretinitis caused by Candida albicans, the most common cause of chorioretinitis, without inducing any significant systemic side effects. Additionally, voriconazole is active against fluconazole-resistant fungi, and according to the report of Shah et al14, this drug is effective for the infection by Scedosporium apiospermum that have become more prevalent. Hence, the role of voriconazole is recommended in the treatment of fungal chorioretinitis. Nevertheless, more clinical trials and experiences will be necessary to prove the agent's efficacy and safety in treating intraocular fungal infection, including more severe cases like fungal endophthalmitis.
Figures and Tables
References
1. Brod RD, Flynn HW Jr, Clarkson JG, et al. Endogenous Candida endophthalmitis. Ophthalmology. 1990. 97:666–674.
2. Dellon AL, Stark WJ, Chetien PB. Spontaneous resolution of endogenous Candida endophthalmitis complicating intravenous hyperalimentation. Am J Ophthalmol. 1975. 79:648–654.
3. Stern GA, Fetkenhour CL, O'Grady RB. Intravitreal amphotericin B treatment of Candida endophthalmitis. Arch Ophthalmol. 1977. 95:89–93.
4. Robertson DM, Riley FC, Hermans PE. Endogenous Candida oculomycosis: report of two patients treated with flucytosine. Arch Ophthalmol. 1974. 91:33–38.
5. Edwards JE Jr, Lehrer RI, Stiehm ER, et al. Severe candidal infections: clinical perspective, immune defense mechanisms, and current concepts of therapy. Ann Intern Med. 1978. 89:91–106.
6. Sande MA, Mandell GL. Gilman AG, Goodman LS, Rall TW, Murad F, editors. Antimicrobial agents: antifungal and antiviral agents. The pharmacological basis of therapeutics. 1985. 7th ed. New York: Macmillan;chap. 54.
7. Marco F, Danes C, Almela M, et al. Trends in frequency and in vitro susceptibilities to antifungal agents, including voriconazole and anidulafungin, of Candida blood stream isolates. Results from a six-year study (1996-2001). Diagn Microbiol Infect Dis. 2003. 46:259–264.
8. Johnson LB, Kauffman CA. Voriconazole: A New Triazole Antifungal Agent. Clin Infect Dis. 2003. 36:630–637.
9. Shah KB, Wu TG, Wilhelmus KR, Jones DB. Activity of Voriconazole against Corneal Isolates of Scedosporium Apiospermum. Cornea. 2003. 22:33–36.
10. Kim SW, Lee EK. A prospective study of intraocular Candidiasis. J Korean Ophthalmol Soc. 1997. 38:368–373.
11. Choi KS, Kim JS, Nam KR. Intraocular Candidiasis in babies with Candida sepsis. J Korean Ophthalmol Soc. 2000. 41:1563–1568.
12. Johnson EM, Szekely A, Warnock DW. In-vitro activity of voriconazole, itraconazole and amphotericin B against filamentous fungi. J Antimicrob Chemother. 1998. 42:741–745.
13. Cuenca-Estrella M, Ruiz-Diez B, Martinez-Suarez JV, et al. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J Antimicrob Chemother. 1999. 43:149–151.
14. Carrillo AJ, Guarro J. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob Agents Chemother. 2001. 45:2151–2153.
15. Espinel-Ingroff A. In vitro fungicidal activities of voriconazole, itraconazole, and amphotericin B against opportunistic moniliaceous and dematiaceous fungi. J Clin Microbiol. 2001. 39:954–958.
16. Meletiadis J, Meis F, Mouton JW. EUROFUNG Network. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob Agents Chemother. 2002. 46:62–68.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download