Abstract
This prospective study was performed to measure the macular and the peripapillary retinal nerve fiber layer (RNFL) thicknesses using optical coherence tomography (OCT) in patients with anisometropic amblyopia. Thirty-one patients with hyperopic anisometropic amblyopia were included. The macular retinal thickness and the peripapillary RNFL thickness were measured using OCT. The mean refractive error was +3.71 diopters (D) and +1.00 D, the mean macular retinal thickness was 252.5 µm and 249.7 µm, and the mean RNFL thickness was 115.2 µm and 109.6 µm, in the amblyopic eye and the normal eye, respectively. OCT assessment of RNFL thickness revealed a significantly thicker RNFL in hyperopic anisometropic amblyopia (P=0.019), but no statistically significant difference was found in macular retinal thickness (P>0.05). In conclusion, the amblyopic process may involve the peripapillary RNFL, but not the macula. However, further evaluation is needed.
Amblyopia is the most frequent cause of unilateral poor visual acuity (VA) in children, with an incidence of 0.5~3.5% in preschool and school-age children.1-7 Amblyopia involves loss of Snellen and grating acuity,8,9 loss of contrast sensitivity,10 and creation of distortions in the shape of a stimulus.11 Amblyopia develops in children up to the age of 6~8 years and persists life-long.
The deleterious effect of amblyopia associated with strabismus, anisometropia and ametropia or occlusion during the neonatal period on the cell growth of the lateral geniculate body has been well established by quantitative histologic studies in several animal species12-15 and in humans.16 However, the initial neural site of the visual deficit in this condition is still under investigation, and studies that have observed the presence of retinal modifications in amblyopic eyes remain inconclusive and controversial.17-19
Optical coherence tomography (OCT) is a noninvasive, noncontact technique that visualizes the retinal structure in vivo with a resolution of 10 to 17 µm,20-21 and can measure the thickness of both peripapillary retinal nerve fiber layer (RNFL) and macula retinal layer. The aim of this study is to compare the macula and peripapillary RNFL thicknesses of the amblyopic eye and the normal eye in patients with anisometropic amblyopia to find the potential initial neural site of the visual deficit in this condition.
This prospective study enrolled 31 patients, aged from 5 to 12 years, with hyperopic anisometropic amblyopia. We examined outpatients who met the following inclusion criteria of hyperopic anisometropic amblyopia: no history or evidence of intraocular surgery, neurologic or retinal disease, glaucoma, nystagmus, and strabismus. The VA difference between the amblyopic and normal eyes was at least 2 lines of Snellen acuity. All patients underwent a detailed eye examination including best corrected VA (BCVA) determination using Snellen chart from 6m distance, manifest refraction and cycloplegic refraction after pupillary dilation with 1% cyclopentolate hydrochloride and 1% tropicamide, alternative cover test, duction and version testing, intraocular pressure (IOP) measurement, slit-lamp biomicroscopy and fundus examination.
In this study, anisometropia was defined as a cycloplegic, spherical equivalent difference greater than 2.00 diopter (D) between fellow eyes. To calculate the mean VA, Snellen VA was converted to logMAR scale, and the mean logMAR VA was reconverted to the Snellen VA. IOP, anterior segment and fundus examination were normal in all eyes.
The macular and RNFL thicknesses were measured by OCT (OCT 3000, version A 3.0; Carl Zeiss-Humphrey system, Dublin, CA, USA). Macular scans, consisting of six radial scans, each scan is 30° apart, around the fovea, were performed to evaluate macular thickness, obtain the retinal thickness from 10 points including the fovea, and construct the 6 mm diameter map (Fig. 1). The mean of these values was used to calculate the average macular thickness. To measure the peripapillary thickness of the nerve fiber layer, circular scans were performed around the optic disc (Figs. 2, 3). Internal fixation was used for macular scanning, and external fixation for optic disc scanning. Multiple images were taken from each eye by an experienced operator.
Retinal thickness was measured as the distance between vitreoretinal interface and the layer corresponding to the pigment epithelium and choriocapillaries, while the foveal thickness was defined as the minimal value located at the image center. The scans were subjected to analysis with standard software provided with the apparatus. Student's two-tail t test was used for data analysis, and a P value of less than 0.05 was considered statistically significant.
The study included 31 unilateral anisometropic amblyopia patients without any other ocular or neurologic disease. There were 16 boys and 15 girls, of mean age 7.7 years (5~12 years), with hyperopic anisometropia (+2.00 D to +6.50 D). Mean corrected VA was 0.29 (0.2 to 0.6) and 1.0 (0.9 to 1.0), and mean refractive error (spherical equivalent of the cycloplegic refraction) was +3.71 D (+2.00 D to +6.50 D) and +1.00 D (emmetropic to +3.00 D) in the amblyopic and normal eyes, respectively (Table 1). Examination of the anterior segment and fundus revealed no abnormalities and IOP was within the normal range.
The macular thickness ranged from 237 to 285 µm (252.5±13.7 µm) in the amblyopic eyes and 230 to 283 µm (249.7±13.3 µm) in the normal eyes. There was no statistically significant difference between the two (P>0.05). The thickness of the peripapillary RNFL ranged from 101 to 142 µm (115.2±9.7 µm) in the amblyopic eyes and 96 to 130 µm (109.6±8.4 µm) in the normal eyes. The former layer was significantly thicker than the latter (P=0.019) (Table 2).
The amblyopic process may have an effect on various levels of the visual pathway. Atrophy involving the cells in the lateral geniculate nucleus that receive input from the amblyopic eye16 has been reported. However, retinal involvement in the amblyopic eye is controversial.17-19 Several experiments have demonstrated that retinal ganglion cells can suffer modifications with light deprivation from birth, including cell loss,9 mean nucleolar volume diminution in ganglion cell cytoplasm and internal plexiform layer thinning in rats and cats,10 and reduction in optic nerve size area in mice.19 Arden and Wooding22 reported that electroretinograms (ERG) elicited by patterned stimuli in humans with various types of amblyopia were significantly reduced. These results suggest that in humans, amblyopia may be associated with changes in retinal function at the level of production of the pattern ERG (PERG), which is presumed to be preganglionic. Other investigators,23,24 on the contrary, have not observed a PERG deficit when optical focus, fixation alignment, and stability were individually optimized.
Recently, several in vivo, structural study techniques such as scanning laser polarimeter (GDx) and OCT have been described and used to evaluate the RNFL and macular thicknesses.
Using a third generation nerve fiber analyzer (GDx; Laser Diagnostic Technologies, San Diego, CA), Colen et al25 measured RNFL thickness in strabismic amblyopia and found no statistically significant difference between the strabismic amblyopic eyes and normal eyes. In the study of Bozkurt et al,26 GDx was performed on 18 anisometropic, 2 strabismic and 4 combined amblyopic eyes and there were no significant differences in the retardation measurements of the nerve fiber layer between amblyopic and normal eyes. However, in a study of using OCT, Yen et al27 reported a thicker RNFL in refractive amblyopia and a significantly different RNFL thickness between the amblyopic and normal eyes in refractive amblyopia patients. They suggested that refractive amblyopia affects the process of postnatal reduction of ganglion cells and that RNFL thickness may be thicker than the normal eye.
In the present study, the peripapillary RNFL and macular thicknesses were measured by OCT and the results showed that the macular thickness was not significantly different between the anisometropic amblyopic eyes and the normal eyes, although the peripapillary RNFL was significantly thicker in the amblyopic eyes. Our results corroborate those of a previous OCT study27 that suggested RNFL is thicker in refractive amblyopia and that the amblyopic process may involve the peripapillary RNFL.
Mrugacz et al28 showed that foveal retinal and RNFL thicknesses were significantly decreased, especially in high myopia. In our study, normal eyes were emmetropic or weakly hyperopic. The average refractive error was +1.00 D, which ensured that the control eye selection was reasonable as the comparison was not with thinner controls.
The average foveal thickness in our study was 183.2 µm in the amblyopic eyes and 178.7 µm in the normal eyes. This result is very similar to that of many other studies using OCT, including Hee et al29 and Kang et al.30 There was no statistically significant difference in average foveal thickness between the two eyes (P>0.05).
In conclusion, peripapillary RNFL was significantly thicker in hyperopic anisometropic amblyopia, whereas the macular thickness was not significantly different between amblyopic and normal eyes. This is the first study to evaluate the macular and foveal thicknesses in anisometropic amblyopic eyes. Our results suggest that although the amblyopic process may not have any significant effect on the macula, it may exert a significant effect on peripapillary RNFL. Further studies, including postmortem, would be instrumental in examining the retinal, histologic and structural differences between amblyopic and normal eyes.
Figures and Tables
 | Fig. 2Optical coherence tomography of retinal nerve fiber layer (RNFL) in a patient with hyperopic anisometropic amblyopia. |
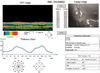 | Fig. 3Optical coherence tomography of retinal nerve fiber layer (RNFL) in the normal eye of a hyperopic anisometropic amblyopic patient. |
References
1. Von Noorden GK. Mechanism of amblyopia. Doc Ophthalmol. 1977. 34:93.
2. Headon MP, Powell TC. Cellular changes in the lateral geniculate nucleus of infant monkeys after suture of the eyelids. J Anat. 1973. 116:135–145.
3. Sherman SM, Wilson JR. Behavioral and morphological evidence for binocular competition in the postnatal development of the dog's visual system. J Comp Neurol. 1975. 161:183–195.
4. Von Noorden GK. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol Vis Sci. 1973. 12:727–738.
5. Wiesel TN, Hubel DH. Effect of visual deprivation on morphology and physiology of cells in the cat's lateral geniculate body. J Neurophysiol. 1963. 26:978–993.
6. Von Noorden GK, Crawford MLJ, Levacy RA. The lateral geniculate nucleus in human anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1983. 24:788–790.
7. Von Noorden GK, Crawford ML. The lateral geniculate nucleus in human strabismic amblyopia. Invest Ophthalmol Vis Sci. 1992. 33:2729–2732.
8. Rasch E, Swift H, Riesen AH, Chow KL. Altered structure and composition of retinal cells in dark-reared mammals. Exp Cell Res. 1961. 25:348–363.
9. Wendell-Smith CP. Effect of light deprivation on the postnatal development of the optic nerve. Nature. 1964. 204:707.
10. Chow KL. Failure to demonstrate changes in the visual system of monkeys kept in darkness or colored light. J Comp Neurol. 1955. 102:597–606.
11. Chauban S, Marshall J. The interpretation of optical coherence tomography image of the retina. Invest Ophthalmol Vis Sci. 1999. 40:2332–2342.
12. Hee MR, Puliafito CA, Wong C. Quantitative assessment of macular edema with optical coherence tomography. Arch Ophthalmol. 1995. 113:1019–1029.
13. Puliafito CA, Hee MR, Lin CP. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995. 102:217–229.
14. DaCunha D, Jenkins EM. Amblyopia in three year olds. Med Officer. 1961. 106:146.
15. Flom MC, Neummaier RW. Prevalence of amblyopia. Public Health Rep. 1966. 81:329.
16. Friedmann Z, Neumann E, Hyams SW, Peleg B. Ophthalmic screening of 38,000 children, age 1 to 2.5 years, in child welfare clinics. J Pediatr Ophthalmol Strabismus. 1980. 17:261–267.
17. Vereecken E, Feron A, Evens L. Importance de la detection prococe du strabisme et de l'amblyopie (in French). Bull Soc Belge Ophthalmol. 1966. 143:729–739.
18. McNeil NL. Patterns of visual defects in children. Br J Ophthalmol. 1955. 39:688–670.
19. Russell EL, Kada JM, Hufhines DM. Orange County vision screening project. Ophthalmologic evaluation. Sight Saving Rev. 1961. 31:215–219.
20. Chow KL, Riesen AH, Newell FN. Degeneration of retinal ganglion cells in infant chimpanzees reared in darkness. J Comp Neurol. 1957. 107:27–42.
21. Rasch E, Swift H, Riesen AH, Chow KL. Altered structure and composition of retinal cells in dark-reared mammals. Exp Cell Res. 1961. 25:348–363.
22. Arden GB, Wooding SL. Pattern ERG in amblyopia. Invest Ophthalmol Vis Sci. 1985. 26:88–96.
23. Hess RF, Baker CL, Nerhoeve JN. The pattern evoked electroretinogram: its variability in normals and its relationship to amblyopia. Invest Ophthalmol Vis Sci. 1985. 26:1610–1623.
24. Deline PJ, Weissenbruch C, Berendschot TT, Norren DV. Photoreceptor function in unilateral amblyopia. Vision Res. 1998. 38:613–617.
25. Colen TP, de Faber JT, Lemij HG. Retinal nerve fiber layer thickness in human strabismic amblyopia. Binocul Vis Strabismus Q. 2000. 15:141–146.
26. Bozkurt B, Irkec M, Orhan M. Thickness of the retinal nerve fiber layer in patients with anisometropic and strabismic amblyopia. Strabismus Binocul Vis Strabismus Q. 2003. 11:1–7.
27. Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004. 45:2224–2230.
28. Mrugacz M, Lazarczyk AB, Kita DS. Use of optical coherence tomography in myopia. J Pediatr Ophthalmol Strabismus. 2004. 41:159–162.
29. Hee MR, Puliafito CA, Duker JS. Topography of diabetic macular edema with optical coherence tomography. Ophthalmology. 1998. 105:360–370.
30. Kang JH, Kim SA, Song WG. Macular thickness changes with age in normal subjects measured by optical coherence tomography. J Korean Ophthalmol Soc. 2004. 45:592–598.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download