Abstract
The aim of this study is to elucidate the association of neovascularization in branch retinal vein occlusion (BRVO) combined with major arterial insufficiency (MAI), compared with BRVO alone. The authors retrospectively reviewed the charts, color photographs, and fluorescein angiograms of 304 patients (308 eyes) who had BRVO from 1990 to 2002 at Hanyang University hospital. Patients with BRVO combined with MAI and patients with BRVO alone were differentiated by angiographic appearance. Of the 308 eyes, 12 (3.9%) had neovascularization, all of which were in the 56 eyes of the MAI group for which the neovascularization rate was 21.4%. Neovascularization in BRVO was more strongly associated with the non-perfusion caused by MAI, rather than with the extent of the non-perfusion area that originated from retinal capillary obstruction. MAI is considered as a risk factor for neovascularization and hence could be a prognostic factor.
Retinal vein occlusion is the most common retinal vascular occlusion disorder and is usually associated with visual loss of variable degree.1,2,3 New vessels occur frequently in this retinal vascular disease. New vessel formation of the retina, which is the most common site, in branch retinal vein occlusion (BRVO) also influences the prognosis of patients more than any other complication, and often leads to recurrent vitreous hemorrhages,2,3 and ultimately to blindness. Most ophthalmologists have known that neovascularization is strongly associated with the extent of capillary non-perfusion area that is more than five disc diameters (DD) in width, as visualized with fluorescein angiography (FAG).2 New vessel formation occurs in response to vasoproliferative substances released by the hypoxic retinal tissue.4-8 There has been general consensus that the wide area of non-perfusion which aggravates tissue hypoxia triggers neovascularization of the retina.9,10
BRVO combined with simultaneous insufficiency of the retinal artery does not yet have a well-defined clinical entity. Its pathophysiologic mechanism may be of fundamental clinical importance due to more frequently induced neovascularization than BRVO alone. In this study, the authors elucidated that BRVO combined with major arterial insufficiency (MAI), compared with BRVO alone, easily induced neovascularization.
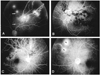
We reviewed the photographs and FAG of 304 patients (308 involved eyes) who had visited our department of ophthalmology at Hanyang University hospital from 1990 to 2002. Fundus photographs and FAG for each patient were reviewed and venous obstruction, non-perfusion and arterial insufficiency (AI) were also evaluated. Medical records were reviewed regarding age, sex, diabetes mellitus, hypertension, visual acuity, intraocular pressure, and any other ocular problems. All of the photographs which had the neovascularization elsewhere (NVE) and neovascularization of the disc (NVD) clearly showed four quadrant areas, the location of AI, the area of non-perfusion and so on. We evaluated whether neovascularization was associated with MAI. No intra-arterial retinal embolism was visualized. Retinal edema, together with hemorrhage, exudates, and microaneurysm, was usually seen within and surrounding the distribution of the occluded vein in both types. We excluded photographs that had serious vitreous hemorrhage disturbing the retinal image and distinctness of FAG. BRVO was defined as delayed venous filling in the area of the occlusion relative to the unaffected retina, narrowing of the retinal venous column draining this area, including dilated and tortuous retinal veins, and retinal hemorrhage along the course of the obstructive vessel. The location of venous obstruction was thought to be the crossing site of the retinal artery obliterating the retinal vein. New vessel formation, such as NVE and NVD, and the area of non-perfusion was also evaluated to identify any association of MAI. Non-perfusion was defined as hypofluorescence that was clearly seen due to vascular filling defect. Patients with BRVO combined with MAI, and patients with BRVO alone were classified according to angiographic appearance. AI was defined as normal branch arterial filling that was not seen or was extremely delayed in FAG. MAI was defined as follows. First, the insufficient portion of the artery started within 2 DD (Fig. 1B). Second, the beginning branch from the main artery showed delayed filling and defect that occurred with the wide area of hypofluorescence between artery and artery, in spite of being collateral vessels (Fig. 1A). We excluded from MAI cases where large branch arteries were filled in the wide area of non-perfusion (Fig. 1C).
Mean age of the BRVO patients (114 men, 190 women) was 54.5 years, ranging between 26 and 77. Two hundred forty-eight patients had BRVO alone, and 56 patients had BRVO combined with MAI. There were 12 cases, all of BRVO combined with MAI, new vessel formation (NVD, NVE) in FAG. Age and sexual differences were not significant factors for new vessel formation. Of the BRVO patients, 126 (40.1%) had systemic hypertension, but no hypertensive retinopathy, 18 (5.92%) had diabetes mellitus, none had diabetic retinopathy, and only 2 patients had open angle glaucoma, which was controlled by medication. Length of follow-up from onset of first episode, which included the first episode of retinal vein occlusion that occurred before the first visit to our department, ranged from 6 to 30 months. The following features of MAI were noted. First, non-perfusion began from the obstructive site of the vein, and the main branch retinal artery filling defect 2 DD distal to the optic disc. The branches of the retinal artery showed delayed filling, but the main artery filled normally (type A) (Fig. 1A). Second, the obstructive site of the vein was located near the optic disc, but non-perfusion began far from the obstructive site. The main artery filled normally, but the branch retinal artery was not perfused (type B) (Fig. 1D). Third, non-perfusion began from the obstructive site of the vein like type A. The site at which the main artery was not perfused was located within 2 DD from the optic disc (type C) (Fig. 1B).
Among the twelve cases of neovascularization, there were four type A, three type B and five type C patients (Table 1). NVE mainly appeared in types A and B, and NVD in type C (Table 1). Only one patient, belonging to type A, had both NVD and NVE. None of them had iris neovascularization. Ten patients showed superior temporal branch obstruction, one had inferior temporal branch obstruction and the other one had superior temporal branch and superior nasal branch obstruction in both eyes. Dilated capillary and microaneurysm was a common sequel to most cases on FAG. Visual disturbance was also associated with vitreous hemorrhage and macular edema, without neovascularization, non-perfusion, or AI. In all cases, the NVE occurred at the border of the perfused and non-perfused retina.
It is not well known how new vessel formation occurs in BRVO, or what factors have an effect on it. To our knowledge, the pathophysiologic mechanism that has been reported is that the high proportion of patients with BRVO which has a wide capillary non-perfusion area, sequestrated by more than 5 DD, further suggests that new vessel formation might be predictive of disease aggravation. In general, among large branch vein occlusion (involving a quadrant or more) cases, about 50% are associated with a large area of capillary non-perfusion, and of these, about 40% will develop neovascularization.2 Furthermore, the clinical significance of macroaneurysm is its association with the ischemic retina as demonstrated by capillary non-perfusion on FAG.11
In this study, we performed analysis of two types of patients with BRVO (BRVO alone, BRVO with MAI) in order to reveal the pathophysiologic mechanism of new vessel formation. In all cases, the neovascularization that occurred at the border of the perfused and non-perfused retina was significantly more marked in BRVO combined with MAI. These twelve patients with neovascularization presented as MAI in conjunction with simultaneous BRVO.
In type A cases, non-perfusion began from the vein obstruction, and the area of non-perfusion became wider than in type B. It is possible that the prognosis was worse than for type B due to the far extent of AI. NVE occurred in 3 cases and NVE, NVD in 1 case.
In type B cases, in spite of the narrow non-perfusion area, MAI caused the neovascularization. Neovascularization might not have occured without MAI, through this case (Fig. 1C). The branch retinal vein of both eyes was not perfused at the same time. Even though the right eye had wider non-perfusion area than the left, the left eye developed NVE first (Fig. 1D). It is apparent therefore, that MAI induced neovascularization, regardless of the extent of the non-perfusion area. This data possibly demonstrates that MAI with a wide area of non-perfusion severely accelerates retinal ischemia. In the Branch Vein Occlusion Study, neovascularization developed in 22% of eyes with BRVO without regard to the degree of non-perfusion.12 We consider that all these cases may have been associated with AI.
In type C cases, there was wide non-perfusion with main AI within 2 DD of the optic disc. All of these cases had NVD. Main branch AI developed prominent ischemia as demonstrated via FAG. In this case, neovascularization may have been formed easily because the perfusion pressure was very low and the hypoxia was severe. We think that NVD might be associated with the extent of AI through these cases.
We concluded by FAG that MAI may be an independent risk factor in BRVO. We have known that the decrease of oxygen tension below normal causes vasodilatation. Retinal vasoconstriction is probably due to high retinal arterial oxygen tension.13-15 However, in these cases vasoconstriction could have occurred in the setting of a decrease in oxygen tension. Moreover this situation is different from BRAO. AI by arterial spasm may be affected by any other factor. First, chemical factors, such as PO2, PCO2, pH, and metabolic products. Donati also showed that experimental BRVO induced in the affected retina an impairment in the release of constitutive NO that contributed to the arterial constriction.16-20 Second, pathologic arterial problems, such as hypertension, and diabetes easily develop AI. Third, severe venous obstruction might affect arterial obstruction according to high hydraulic pressure.
In 1990, Duker and coworkers reported that five of seven patients with the unusual combination of a central retinal vein occlusion (CRVO) in conjunction with a simultaneous BRAO suffered markedly diminished visual function, and that two eyes developed prominent retinal ischemia which revealed widespread peripheral capillary non-perfusion.21
Hayreh indicated that the hemorrhagic retinopathy seen in occlusive vascular disorders is due to combined occlusion of the central retinal artery and vein ,and is not due to venous occlusion solely.22 He also reported that CRVO with central retinal artery obstruction leads to stasis of the retinal circulation which in turn produces venous stasis, acting as a triggering factor to complete the occlusion in the vein.23 We thought that BRVO occurring in conjunction with AI was also a similar case. AI accelerates venous stasis in BRVO.
AI and venous stasis interact to drive the perfusion pressure lower, which induces tissue hypoxia. Until now, many ophthalmologists have studied the non-perfusion related to neovascularization in the case of BRVO, but we think that BRVO was also associated with AI like CRVO.
Our research demonstrated that main branch AI near the optic disc and severe AI are more important in the formation of neovascularization than the area of non-perfusion. Neovascularization of the retina was prominent in most cases of BRVO with MAI examined on FAG.
The following question remains: why would retinal artery insufficiency aggravate neovascularization? While there is no doubt that experimental branch vein occlusion produced the areas of ischemia, it cannot be concluded that there was significant retinal hypoxia, which the induction of neovascularization depends upon.9,10,24 No retinal changes on CRVO occurred, whereas hemorrhage with destruction of the inner retinal layer on simultaneous occlusion of the artery and vein was observed in a histological examination of the rhesus monkey.25 In 1991 Schatz and associates suggested that CRVO produces an elevation of luminal capillary pressure, because the central retinal artery continues to pump blood into the retina. At this time, the perfusion pressure of the central retinal artery is usually slightly higher than that of the perfusion pressure of the posterior ciliary artery circulation.26 We think its role is also attributed to BRVO. If arterial blood flow is sufficient for perfusion pressure with a wide area of non-perfusion, retinal tissue will not fall into hypoxia. Furthermore, the high metabolic requirement of the retina is prone to be influenced by the effects of blood flow change.27 Collateral circulation played the important role of a pressure gradient sufficient for capillary perfusion in venous obstruction.9 Even if the occluded vein with MAI was recanalized by collateral vessels, it might be insufficient for capillary perfusion. In conclusion, the mechanism involves MAI leading to lower capillary pressure. As a result, new vessels were only occasionally observed in retinas with AI known to cause extended ischemia, since the induction of neovascularization depends on the degree of hypoxia.
These results may elucidate that the decrease of oxygen partial pressure triggers neovascularization in BRVO. For this reason, BRVO combined with retinal AI has more serious problems than BRVO alone.
Causal mechanisms have been proposed for BRVO with MAI. First, the severity of venous obstruction might affect arterial obstruction according to high hydraulic pressure. In this case, severe venous obstruction can decrease the perfusion pressure in AI. Second, retinal blood flow is autoregulated by a myogenic mechanism, whereas factors related to the retinal metabolism (retinal tissue PO2, PCO2, pH, and metabolic products) affect the retinal arterial tone.9 Donati also showed that experimental BRVO induced in the affected retina with impairment in the release of constitutive NO contributed to the arterial constriction.16-20 The AI caused by these two mechanisms deteriorates venous stasis. The AI caused by this condition simultaneously affects tissue hypoxia in regard to lower perfusion pressure. As a result, AI is the decisive factor for neovascularization. Third, there are a lot of arterial problems as demonstrated by the ease with which MAI developed in the BRVO patients with hypertension and diabetes mellitus. However, our study showed that only two patients had hypertension and diabetes with retinal neovascularization. Therefore the status of arterial problem is not always crucial. Fourth, although the severity of venous obstruction can induce the proliferation of neovascularization, lowering capillary perfusion pressure in AI is fundamentally the strongest causative factor. Fifth, the combination of AI in a large artery and a more proximal arterial obstruction to the optic disc developed extremely low perfusion pressure, thereby worsening the prognosis.
If this bad condition had been controlled, the retinal artery would have returned to a normal state without permanent AI. The retina was exposed to non-perfusion, and subsequently arterial spasm occurred. Finally neovascularization may develop.
A limiting factor of this study is that we couldn't exactly determine the results of those patients who did not have neovascularization with MAI, because these patients were not continuously followed up and they were treated by laser photocoagulation in the middle of follow up. The two types of BRVO could not be clearly categorized by the initial examination. We generally have not used laser photocoagulation in BRVO patients until neovascularization occurs. However, if BRVO with MAI is detected, prompt laser photocoagulation should be applied.
Figures and Tables
Fig. 1
(A) Arterial narrowing and filling defect is visible (white arrows). NVE is located on the vein(black arrowhead). (B) FAG shows that the arterial filling defects start within proximal to 2 DD (white arrows). Prominent neovascularization is located on the optic disc (black arrowheads). (C) Many branch arteries are filled in wide area of non-perfusion. (D) The branch artery has a filling defect (arrow). NVE (black arrowheads) is located between the artery and vein.
References
1. The Eye Disease Case-control Study Group. Risk factors for branch retinal vein occlusion. Am J Ophthalmol. 1993. 116:286–296.
2. Ryan SJ. Retina. 1989. 2nd ed. St. Louis: Mosby;1387–1392.
3. Frederick A. Principles and practice of ophthalmology vol 2: retina and vitreous. 1994. 1st ed. Philadelphia, Pennsylvania: Saunders;740–743.
4. Patz A. Retinal neovascularization: early contributions of professor Michaelson and recent observation. Br J Ophthalmol. 1984. 68:42–46.
5. Michaelson IC. The mode of development of the vascular system of the retina with some observation on its significance for certain retinal diseases. Trans Ophthalmol Soc UK. 1948. 68:137–180.
6. Wise GN. Retinal neovascularization. Trans Am Ophthalmol. 1956. 54:729–826.
7. Henkind P. Ocular Neovascularization. Am J Ophthalmol. 1978. 85:287–301.
8. Ben Ezra D. Neovasculogenesis. Triggering factors and possible mechanism. Surv Ophthalmol. 1979. 24:167–176.
9. Constantin J. Retinal oxygen distribution, its role in the physiopathology of vasoproliferative microangiopathies. Retina. 1995. 15:332–347.
10. Hamilton AM, Marshall J, Kohner EM, Bowbyes JA. Retinal new vessel formation following experimental vein occlusion. Exp Eye Res. 1975. 20:493–497.
11. Scott WC, Harry WF, John GC. Macroaneurysms associated with retinal branch vein occlusion. Am J Ophthalmol. 1990. 109:567–570.
12. The Branch Vein Occlusion Study Group. Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion. Arch Ophthalmol. 1986. 104:34–41.
13. Deutsch RA, Read JS, Ernest JT. Effects of oxygen and carbon dioxide on the retinal vasculature in humans. Arch Ophthalmol. 1983. 101:1278–1280.
14. Frayser R, Hickam JB. Retinal vascular response to breathing increased carbon dioxide and oxygen concentrations. Invest Ophthalmol Vis Sci. 1964. 3:427–431.
15. Riva CE, Grunwald JE, Sinclair SH. Laser Dopplervelocimetry study of the effect of pure oxygen breathing on retinal blood flow. Invest Ophthalmol Vis Sci. 1983. 24:47–51.
16. Donati G, Pournaras CJ, Tsacopoulos M. Effect of nitroprusside on arteriolar constriction after retinal branch vein occlusion. Invest Ophthalmol Vis Sci. 1998. 39:1910–1917.
17. Donati G, Pournaras CJ, Pizzolato GP, Tsacopoulos M. Decrease nitric oxide production accounts for secondary arteriolar constriction after retinal branch vein occlusion. Invest Ophthalmol Vis Sci. 1997. 38:1450–1457.
18. Donati G, Pournaras CJ, Munoz JL. Nitric oxide controls arteriolar tone in the retina of the miniature pig. Invest Ophthalmol Vis Sci. 1995. 36:2228–2237.
19. Pournaras CJ, Tsacopoulos M, Strommer K. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990. 97:1321–1328.
20. Pournaras CJ. Retinal oxygen distribution. Its role in the physiopathology of vasoproliferative microangiopathies. Retina. 1995. 15:332–347.
21. Duker JS, Cohen MS, Brown GC. Combined branch retinal artery and central retinal vein obstruction. Retina. 1990. 10:105–112.
22. Hayreh SS. Pathogenesis of occlusion of the central retinal vessels. Am J Ophthalmol. 1971. 72:998–1011.
23. Hayreh SS. So-called central retinal vein occlusion. Ophthalmologica. 1976. 172:1–13.
24. Kohner EM, Dollery CT, Shakib M, et al. Experimental retinal branch vein occlusion. Am J Ophthalmol. 1970. 69:778–825.
25. Hayreh SS. Occlusion of the central retinal vessels. Br J Ophthalmol. 1965. 49:626–645.
26. Schatz H, Fong A, McDonald R, Johnson R. Cilioretinal artery occlusion in young adults with central retinal vein occlusion. Ophthalmology. 1991. 98:594–601.
27. Ariturk N, Oge Y, Erkan D. Relation between retinal vein occlusion and axial length. Br J Ophthalmol. 1996. 80:633–636.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download