Abstract
This study was performed to compare the incidence of posterior capsular opacity (PCO) and refractive errors between hydrophilic (ACR6D, Corneal®) and hydrophobic (MA60BM, AcrySof®) acrylic intraocular lenses (IOLs) over a 3-year follow-up after phacoemulsification surgery. The patients with AcrySof® implanted in one eye and Corneal® in the other eye were categorized as Group 1 (n=28), while those with one or both eyes implanted with IOLs of the same kind were categorized as Group 2 (AcrySof®, n=90; Corneal®, n=95). Refractive errors were evaluated at 3 months and 3 years postoperatively. The incidence of visually significant PCO was investigated 3 years postoperatively. Postoperative refractive values at 3 months were not significantly different between the two groups. However, refractive values at 3 years were significantly different between two IOLs in both groups [AcrySof® -0.37±0.43D, Corneal® -0.62±0.58D in Group 1 (p=0.04); AcrySof® -0.38±0.52, Corneal® -0.68±0.54 in Group 2 (p<0.01)]. The incidence of visually significant PCO was 14% and 32% in Group 1, and 13% and 28% in Group 2, for the AcrySof® and Corneal® implants, respectively. The incidence of visually significant PCO of hydrophilic acrylic IOLs was higher than that of hydrophobic acrylic IOLs in the 3-year follow-up. The postoperative 3-year refractive value of Corneal® showed myopic shift.
Although advances in cataract surgery have reduced the incidence of complications, posterior capsular opacity (PCO) has been frequently reported as one of the major complications with an incidence rate ranging from 1.5% to 55.6%, depending on the types of intraocular lens (IOL) and the period of follow-up examination.1-3 Although many studies have claimed that the material and design of IOL have an important impact on the incidence of PCO, the main cause of PCO remains unclear. Many studies have dealt with hydrophilic and hydrophobic acrylic IOLs, showing high biocompatibility and low incidence of PCO, but fewer studies have compared the clinical consequences of these two lenses on a long-term basis.4-10
The present study aimed to investigate the incidence of PCO and refractive errors of hydrophilic (ACR6D, Corneal®) and hydrophobic (MA60BM, AcrySof®) foldable acrylic IOLs over a three-year follow-up period after phacoemulsification surgery.
The medical records of patients who had undergone phacoemulsification and posterior chamber lens implantation performed by one surgeon at the Department of Ophthalmology of Korea University Hospital between May 1998 and August 2000 were analyzed retrospectively. The 28 patients with AcrySof® implanted in one eye and Corneal® in the other eye were categorized as Group 1 and those implanted with only one kind of IOL in either one eye or both eyes were categorized as Group 2. In Group 2, AcrySof® was implanted in 90 eyes and Corneal® was implanted in 95 eyes. The study excluded those who had posterior capsular rupture during surgery, cystoid macular edema after surgery or complicated cataract caused by trauma and uveitis, and those who had a history of ocular disease including diabetic retinopathy, glaucoma and prior history of ocular surgery. Those who were followed up for less than 3 years were also excluded.
All cataract surgeries were performed by the same surgeon using phacoemulsification. Corneal anaesthesia was achieved with 2 to 3 drops of propacaine (0.5%). After a clear corneal incision was made, continuous curvilinear capsulorhexis was performed and the cataract was removed using ultrasound phacoemulsification. As many lens epithelial cells were removed as possible using automated irrigation and aspiration device, after which foldable AcrySof® and Corneal® lenses were implanted and positioned into the capsular bag. A clear corneal incision was closed with 10-0 nylon suture. After surgery, all patients were guided to use ofloxacin 0.3% and prednisolone 1% eye drops four times a day for three weeks. The target lens power ranged from plano to -0.5 D. Although the A constant of AcrySof® (118.9) was used without adjustment, the A constant for Corneal® was decreased by 0.5 (118.5), given the findings of earlier studies showing that the use of the original A constant for Corneal® lens (119.0) frequently resulted in myopia.
Visually significant PCO was defined as a loss of 3 or more lines of best corrected visual acuity in Snellen chart or when the patient reported reduced visual acuity.
The study compared the refractive errors measured 3 months and 3 years after surgery and investigated the incidence of visually significant PCO and the use of Nd:YAG laser posterior capsulotomy.
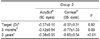
The mean age was 64.68±12.16 years in Group 1, 63.84±10.03 in patients implanted with AcrySof® in Group 2 and 65.46±14.21 years in patients implanted with Corneal® in Group 2, showing no significant difference (p>0.05)(Table 1). In eyes implanted with AcrySof® in Group 1, the target lens diopter was -0.38±0.15D, while the refractive error was -0.40±0.62D 3 months after surgery and -0.37±0.43D 3 years after surgery. In eyes implanted with Corneal® in Group 1, the target lens diopter was 0.36±0.13D, while the refractive error was -0.56±0.67D 3 months after surgery and -0.65±0.58D 3 years after surgery. There were no significant differences in preoperative target diopter or the refraction measured 3 months after surgery between the two subgroups (p=0.45, p=0.09). But the refraction at 3 years postoperatively was significantly different in the two subgroups with the patients implanted with Corneal® showing myopic shift (p=0.04). The difference between target diopter and postoperative refractions was not significant in patients implanted with AcrySof® (p>0.05). However, the diopter values were significantly lower in patients implanted with Corneal® in the two post-surgery assessments, compared with the preoperative target diopter (P=0.03, 0.01) (Table 2-1). In eyes implanted with AcrySof® in Group 2, the target lens diopter was -0.38±0.15D, while the refractive error was -0.40±0.62D 3 months after surgery and -0.37±0.43D 3 years after surgery. In eyes implanted with Corneal® in Group 2, the target lens diopter was -0.36±0.13D, while the refractive error was -0.56±0.67D 3 months after surgery and -0.65±0.58D 3 years after surgery. Though no significant differences were found in target diopter and 3-month postoperative refractive error between these two subgroups of Group 2 (p=0.82, 0.09), the 3-year postoperative refractive error in those implanted with Corneal® indicated a tendency for myopic shift (p<0.01). There was no statistically significant difference between target diopter and postoperative refractions in those implanted with AcrySof®, but the 3-year post-surgery diopters in those implanted with Corneal® were significantly lower than those values measured prior to surgery and 3 months after surgery (p=0.003, 0.04, respectively) (Table 2-2).
Among those implanted with AcrySof®, 4 eyes (14%) of Group 1 and 12 (13%) of Group 2 developed visually significant PCO. Among those implanted with Corneal®, 9 eyes (32%) of Group 1 and 27 (28%) of Group 2 developed visually significant PCO (Table 3).
To remove the opacity of the posterior capsule, Nd:YAG capsulotomy was performed in 2 (7.1%) and 6 (6.6%) eyes, among those implanted with AcrySof® in Groups 1 and 2, respectively, and in 7 (25%) and 18 (18.9%) eyes, among those implanted with Corneal® in Groups 1 and 2, respectively (Table 4).
As for the characteristics of the two IOLs used for the study, AcrySof® MA60BM, a hydrophobic acrylic IOL, is a proprietary copolymer of phenylethyl acrylate and phenylethyl methacrylate with an optic diameter of 6.0 mm and a rectangular optic edge with angulated modified C monofilament haptics. Corneal® ACR6D, a hydrophilic acrylic IOL, is a proprietary copolymer of 2-hydroxyethyl methacrylate and methylmethacrylate with a 6.0 mm optic diameter and single piece-loop haptics without angulation. The overall length is 13.0 mm and 12.5 mm, respectively, for MA60BM and ACR6D.
The study found that those implanted with the hydrophilic acrylic IOL had a higher incidence of PCO. This result supported the findings of earlier studies that the materials of IOLs are a major contributory cause of PCO. Several studies suggested that the incidence of PCO was lower in eyes implanted with IOLs made from acryl than with those made from PMMA and silicone polymer.3,8,9,11,12 Sundelin et al2 reported a 6.2% incidence rate of PCO during a 3-year follow-up examination. Ram et al3 reported a 6.5% incidence rate of PCO and that Nd:YAG laser capsulotomy was performed to remove the opacity. Scaramuzza et al1 said that 55.6% of patients implanted with hydrophilic acrylic IOLs needed a capsulotomy in the presence of PCO during a 13.1-month follow-up period. Hydrophilic acrylic IOLs are known for their high degree of biocompatibility, which in turn suppresses disruption to blood-aqueous barriers and eventually reduces inflammation in the eye. While some investigators claimed that the inflammatory response stimulated the proliferation and migration of lens epithelial cells, other investigators such as Hollick et al14 asserted that a high degree of IOL biocompatibility could induce lens epithelial cell proliferation after finding greater proliferation of lens epithelial cells as the damage in blood-aqueous barriers and the inflammation responses in the eye were smaller. Thus the relationship between lens biocompatibility and the incidence of PCO still sparks debate among investigators.13-16
The design of IOLs is also cited as the major contributory cause of PCO. AcrySof® has a sharp optic edge while Corneal® has a round optic edge. Nishi and Nishi10 urged that IOLs with a sharp optic edge design are more effective to suppress the migration of lens epithelial cells and eventually reduce the incidence of PCO by supporting the formation of a capsular bend at the optic edge. Several studies have supported Nishi and Nishi's findings.17,18 Some studies reported no difference in the effects of silicone and acrylic lens with a sharp optic edge on the opacities of the anterior and posterior capsules and on lens epithelial cell proliferation. These studies therefore concluded that the sharpness of the optic edge of the lens is a more significant factor in preventing PCO than the material of the lens. Other studies also stressed that the degree of bend formation and speed are important to prevent PCO, regardless of the sharpness of the optic edge.19,20
Separately, AcrySof® has monofilament haptics while Corneal® has loop haptics. Nishi and Nishi21 stated that lenses with bulky haptics might raise the incidence of PCO by hampering capsular bend formation.
The corneal refractive power remained stable in those patients implanted with AcrySof® in both Groups 1 and 2, resulting in no significant changes in preoperative target diopter value or refractive error at 3 months and 3 years after surgery. Those implanted with Corneal® in both Groups 1 and 2, however, showed significant myopic refractive error after surgery, compared with preoperative target value, despite the fact that the refractive power was modified to match a hyperopic level by reducing A constant for this lens by 0.5. In addition, eyes implanted with Corneal® in Group 1 experienced an increase in myopic refractive value at 3 years after surgery compared to 3 months after surgery. Earlier studies reported dislocation of IOL from the center, and backward or forward migration of IOL from the iris plane due to changes in zonular fiber configuration in soft, one-piece IOLs.22,23 One of the probable causes of myopic shift in Corneal® implanted eyes was the anterior migration of the IOL-capsular bag complex as capsular adhesion and fibrosis progressed. Further studies are necessary to compare temporal changes in the distance between the cornea and the IOL. A possible limitation of the present study is that the A constant for this lens may have been incorrectly decided. Therefore, further studies are necessary to ensure the effects of reduced capsular adhesion on the development of myopic shift using the new hydrophilic acrylic IOLs that have been recently developed for reducing the occurrence of PCO, capsular adhesion and fibrosis.
Lens calcification, previously reported in eyes implanted with hydrophilic acrylic Hydroview® IOLs, was not found in this study over the 3-year follow-up.24,25
To minimize individual patient bias, patients of Group 1 had AcrySof® implanted in one eye and Corneal® in the other eye. However, the sample size of Group 1 was too small to draw a conclusion on the incidence of PCO, thereby prompting the authors to form a second group, Group 2, with an increased number of study subjects in order to compare data results.
It is concluded that the materials of IOLs and the design of optic and haptics combine to play a crucial role in triggering PCO, although specific reasons for this effect remain unclear. Further studies are necessary to determine the effects of newly developed, hydrophilic acrylic IOLs with a rectangular edge on PCO occurrence, refractive change and distance between the cornea and IOLs.
References
1. Scaramuzza A, Fernando GT, Crayford BB. Posterior capsule opacification and lens epithelial cell layer formation: Hydroview hydrogel versus AcrySof acrylic intraocular lenses. J Cataract Refract Surg. 2001. 27:1047–1054.
2. Sundelin K, Friberg-Raid Y, Ostberg A, Sjostrand J. Posterior capsular opacification with AcrySof and polymethylmethacrylate intraocular lenses. J Cataract Refract Surg. 2001. 27:1586–1590.
3. Ram J, Kaushik S, Brar GS, Gupta A. Neodymium:YAG capsulotomy rates following phacoemulsification with implantation of PMMA, Silicone, and Acrylic intraocular lenses. Ophthalmic Surg Lasers. 2001. 32:375–382.
4. Hayashi K, Hayashi H, Nakao F, Hayashi F. Reduction in area of the anterior opening after polymethylmethacrylate, silicone and soft acrylic intraocular lens implantation. Am J Ophthalmol. 1997. 123:441–447.
5. Ursell PG, Spalton DJ, Pande MV. Anterior capsular stability in eyes with intraocular lenses made of polymethylmethacrylate, silicone and AcrySof. J Cataract Refract Surg. 1997. 23:1532–1538.
6. Nagata T, Minakata A, Watanabe I. Adhesiveness of AcrySof to a collagen film. J Cataract Refract Surg. 1998. 24:367–370.
7. Gabriel MM, Aheam DG, Chan LY, Patel AS. In vitro adherence of Pseudomonas aeruginosa to four intraocular lenses. J Cataract Refract Surg. 1998. 24:124–129.
8. Ursell PG, Spalton DJ, Pande MV, et al. Relationship between intraocular lens biomaterials and posterior capsular opacification. J Cataract Refract Surg. 1998. 24:352–360.
9. Barret GD, Constable IL, Stewart AD. Clinical results of hydrogel lens implantation. J Cataract Refract Surg. 1986. 12:623–631.
10. Nishi O, Nishi K. Preventing posterior capsular opacification by creating a discontinuous sharp bend in the capsule. J Cataract Refract Surg. 1999. 25:521–526.
11. Hollick EJ, Spaton DJ, Ursell PG, et al. The effect of polymethylmethacrylate, silicone, and polyacrylic intraocular lenses on posterior capsular opacification 3 years after cataract surgery. Ophthalmology. 1999. 106:49–54.
12. Hollick EJ, Spalton DJ, Ursell PG, Pande MV. Biocompatibility of poly(methylmethacrylate), silicone and AcrySof intraocular lenses: randomized comparison of the cellular reaction on the anterior lens surface. J Cataract Refract Surg. 1998. 24:361–366.
13. Schauersberger J, Kruger A, Abela C, et al. Course of postoperative inflammation after implantation of 4 types of foldable intraocular lenses. J Cataract Refract Surg. 1999. 25:1116–1120.
14. Hollick EJ, Spalton DJ, Ursell PG. Surface cytologic features on intraocular lenses: can increased biocompatibility have disadvantages? Arch Ophthalmol. 1999. 117:872–878.
15. Nishi O, Nishi K, Imanishi M. Synthesis of interleukin-1 and prostaglandin E2 by lens epithelial cells of human cataracts. Br J Ophthalmol. 1992. 76:338–341.
16. Nishi O, Nishi K, Fujiwara T, et al. Effects of the cytokines on the proliferation of and collagen synthesis by human cataract lens epithelial cells. Br J Ophthalmol. 1996. 80:63–68.
17. Peng Q, Visessook N, Apple DJ, et al. Surgical prevention of posterior capsular opacification. Part 3: intraocular lens optic barrier effect as a second line of defense. J Cataract Refract Surg. 2000. 26:198–213.
18. Buehl W, Findl O, Menapace R, et al. Effect on an acrylic intraocular lens with a sharp posterior optic edge on posterior capsular opacification. J Cataract Refract Surg. 2002. 28:1105–1111.
19. Schauersberger J, Amon M, Kruger A, et al. Comparison of the biocompatibility of 2 foldable intraocular lenses with sharp optic edges. J Cataract Refract Surg. 2001. 27:1579–1585.
20. Nishi O, Nishi K. Preventive effect of a second-generation silicone intraocular lens on posterior capsular opacification. J Cataract Refract Surg. 2002. 28:1236–1240.
21. Nishi O, Nishi K. Effect of the optic size of a single-piece acrylic intraocular lens on posterior capsule opacification. J Cataract Refract Surg. 2003. 29:348–353.
22. Neumann AC, Cobb B. Advantages and limitations of current soft intraocular lenses. J Cataract Refract Surg. 1987. 15:257–263.
23. Kim JY, Cha HW. The clinical results of hydrophilic acrylic lens. J Korean Ophthalmol Soc. 2001. 42:1562–1570.
24. Yu AK, Kwan KY, Chan DH, Fong DY. Clinical features of 46 eyes with calcified hydrogel intraocular lenses. J Cataract Refract Surg. 2001. 27:1596–1606.
25. Werner L, Apple DJ, Escobar-Gomez M, et al. Postoperative deposition of calcium on the surfaces of a hydrogel intraocular lens. Ophthalmology. 2000. 107:2179–2185.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download