Abstract
Purpose
To evaluate the efficacy of porous expanded polytetrafluoroethylene (e-PTFE, Goretex®) containing large pores made with a 21-gauge needle as a graft for the correction of lower lid retraction.
Methods
e-PTFE grafts were implanted between the tarsus and lower lid retractor via a transconjunctival approach with/without amniotic membrane transplantation, or via a transcutaneous approach. Rabbits were examined and assessed for corneal and conjunctival complications and for e-PTFE graft status. Rabbits were sacrificed for a histological study at 8 weeks postoperatively.
Results
e-PTFE grafts were uniformly extruded 3 weeks postoperatively in eyelids operated on via the transconjunctival approach. However, rabbits operated on via the transcutaneous approach demonstrated e-PTFE graft retention; in addition, dense fibrovascular ingrowths into the large pores of e-PTFE were observed histologically.
Commonly associated with Graves' ophthalmopathy, lower lid retraction can develop after orbital or lid surgery.1-3 This may result in ocular discomfort, exposure keratitis and unacceptable cosmesis. Its surgical correction frequently requires a spacer graft between the tarsus and the lower lid retractor. Homologous sclera, autogenous cartilage and hard palate mucous membrane are commonly used as spacer materials, each with its own drawbacks. Absorption and the recurrence of eyelid retraction for homologous sclera,4-6 additional surgical manipulation to obtain autogenous cartilage7 and hard palate8,9 require new synthetic materials. Expanded polytetrafluoroethylene (e-PTFE) (Gortex, Gore and Associates, Flagstaff, AZ) is an alloplastic material with many uses in reconstructive surgery.10-14 This material is inert, highly biocompatible, mechanically strong and is well tolerated by surrounding tissues, inciting little inflammatory response. In addition, it has a porous structure which is associated with capillary and connective tissue ingrowth.15,16 However, a significant drawback to using this material as a spacer for lower lid retraction occurs when one side of the implanted e-PTFE sheet is exposed, the exposed area is not subsequently covered by conjunctival or fibrous tissue and the e-PTFE sheet is eventually extruded.15 This study was conducted to validate two hypotheses: 1) Artificial large pores made with a 21 gauge needle over the e-PTFE will induce rapid fibrovascular ingrowth and anchor the e-PTFE firmly to the surrounding tissue; 2) When transplanted over the exposed e-PTFE, the amniotic membrane will facilitate conjunctival overgrowth. In addition, we attempted to select an appropriate surgical procedure for implanting e-PTFE as a lower lid spacer.
e-PTFE sheet (1mm thick; 20-30 µm pore size) was cut into 15 mm×4 mm strips and was repeatedly perforated using a 21-gauge needle (Fig. 1).
Thirty New Zealand white rabbits (2.5 kg) were used in this study in accordance with the guidelines established by the Association for Research in Vision and Ophthalmology (ARVO). Anesthesia was induced by an intramuscular injection of ketamine (40 mg/kg) and xylazine (6 mg/kg). Three methods were used to implant e-PTFE spacers; (a) Group A (10 lids) - a transconjunctival approach was used to detach the lower eyelid retractor complex from the inferior tarsal border (Fig. 2A). The implant was then sutured to the inferior tarsal margin superiorly and to the lower eyelid retractor inferiorly using an interrupted 6-0 polygalactin suture (Fig. 2B), (b) Group B (10 lids) - after spacer implantation through a transconjunctival incision, amniotic membrane was used to cover the e-PTFE spacer (Fig. 2C), (c) Group C (10 lids) - a transcutaneous approach was used to create a skin muscle flap, open the septum, and separate the lower eyelid retractor from the lower tarsal border (Fig. 3A). After anchoring the e-PTFE spacer with sutures, the skin wound was closed with a running 6-0 polygalactin suture (Fig. 3B). Tarsorrhaphy was performed for 1 week to immobilize and stretch the lower lids in all cases. Postoperatively, all animals received ofloxacin ophthalmic ointment twice daily. Weekly examinations of the surgical wounds, e-PTFE spacer, conjunctiva, and cornea were performed. Rabbits that demonstrated spacer retention at 8 weeks were sacrificed and the lower eyelids were completely excised, fixed in formalin, embedded in paraffin, stained with hematoxylin and eosin and then studied histopathologically.
The rabbits in group A, in which the corneal side of the e-PTFE spacers was left exposed, showed conjunctival injection and corneal opacity at 1 week after spacer implantation (Fig. 4). These spacers were uniformly extruded 3 weeks after surgery. The amniotic membrane used to cover the exposed surface of the e-PTFE (group B) had melted down completely at 1 week postoperatively. There was no evidence of conjunctival overgrowth or migration onto the surface of the amniotic membrane. Conjunctival injection and corneal opacity were observed at 2 weeks after implantation and e-PTFE spacers were extruded at 3 weeks postoperatively. In group C, 10 lids showed no spacer extrusions, and no conjunctival or corneal complications occurred during the period from placement to sacrifice for histopathological examination (at 8 weeks postoperatively).
The results of the histopathological examination of the e-PTFE demonstrated apparent ingrowth of fibrous tissue and cellular infiltration into the micro-pores that was limited to the surface area of the e-PTFE (Fig. 5A). Contrary to the micro-pores, dense fibrovascular tissue ingrowth was observed in the large pores made using a 21-gauge needle (Fig. 5B).
Lower eyelid position is affected by a number of factors.3 Gravity and the position of the globe relative to the lower lid are two important considerations. In the presence of significant horizontal laxity, the lower eyelid may be tightened by a number of eyelid-shortening procedures. The surgical techniques and materials used to correct lower eyelid retraction must be tailored with respect to the cause of the retraction and with respect to the lamella involved. Skin grafts and rotational flaps are used to correct anterior lamellar deficiencies, whereas interpositional grafts and recession of the eyelid retractors are used for posterior lamellar abnormalities.1 Inferior retractor recession or extirpation of the lower lid retractor may be used for mild cases, but these are ineffective when lower lid retraction exceeds 2 mm.17,18 In addition, spacers must be used for more severe retraction of the lower eyelid.
Ideal spacers must have thickness, rigidity and contour characteristics that approximate the lower lid tarsus, a mucosal surface, so as not to irritate the corneal surface and have no risk of rejection or absorption.
Donor sclera is useful for lower lid lengthening, but tends to be associated with recurrent retraction due to graft absorption and fibrosis4-6 and homologous grafts carry a risk of disease transmission. Autogenous grafts including ear cartilage, hard palate and tarsal plate are limited by the amount of donor material available, donor site morbidity and increased operating time for graft harvesting.7-9 Therefore, searches have been conducted for synthetic graft materials that avoid some of these drawbacks and are easy to obtain and well tolerated.
Expanded PTFE graft material has a unique porosity. Its micro-pores (sized 20-30 µm) can allow fibrovascular tissue ingrowth and an interweaving of PTFE fibrils provides the graft material high tensile strength and minimal deformation under load.19-21 PTFE is chemically and biologically inert, non-antigenic, is easily cut, molded, sutured and is resistant to infection. For these reasons, it has been routinely used as a vascular and abdominal patch graft. Recently, it was introduced to ophthalmic reconstructive surgery and its usefulness as a wrapping material for anophthalmic implants,22 as a substitute for mucous membrane,11 as an interpositional graft material for lower eyelid retraction10 and as a sling material12 has been reported.
There are two disadvantages of e-PTFE when used as a spacer graft to correct lower eyelid retraction. First, the pore size of e-PTFE (20-30 µm) is insufficient to allow the rapid ingrowth of fibrovascular tissue. Fibrovascular ingrowth into the PTFE matrix is very important because it anchors the e-PTFE graft in place and e-PTFE is more resistant to infection when fully vascularized. Second, the exposed areas of e-PTFE cannot support the overgrowth of conjunctival or fibrous tissue, which results in the extrusion of the e-PTFE graft.15 We attempted to overcome these two problems by artificially creating large pores and by covering the exposed e-PTFE with amniotic membrane. Amniotic membrane consists of a basement membrane and an avascular stromal matrix; the basement membrane facilitates epithelial migration, adhesiveness and differentiation.23 Moreover, amniotic membrane has been shown to be effective as a functional substrate for the proliferation and differentiation of epithelial cells in ocular surface reconstruction. To date, preserved human amniotic membrane has been shown to be an effective alternative to conjunctival flaps in the treatment of persistent corneal epithelial defects,24 in pterygium surgery25 and for conjunctival surface reconstruction after the removal of tumors, scarring, symblepharon26 and in entropion surgery.27 We modified the surface of the PTFE graft with amniotic membrane hoping that it would facilitate conjunctival overgrowth, improve graft acceptance and protect the cornea from the graft surface during wound healing.
In these experiments, as observed histologically, e-PTFE graft materials implanted via a transcutaneous incision demonstrated consistent retention and fibrovascular ingrowths. Large pores made by a 21-gauge needle induced the rapid incorporation of fibrovascular tissue into the graft matrix and helped anchor the graft in place. However, fibrovascular ingrowth through micro-pores was protracted and occurred only to a limited extent at 8 weeks postoperatively. Unlike the transcutaneous incision, graft materials implanted via a transconjunctival incision invariably extruded whether covered with amniotic membrane or not. Amniotic membranes were unable to support conjunctival overgrowth or prevent this graft extrusion. This may have been caused by the negatively charged surface of the hydrophobic PTFE fibrils and by the pore size of the woven PTFE sheet utilized in these studies. Incomplete fibrovascularization through micropores and a lack of adhesion between the amniotic membrane and the e-PTFE graft material most likely contributed to the graft extrusion. Moreover, e-PTFE sheets used in the eyelid are a part of a dynamic structure; each blink of the eye generates a shearing force,28 which may differentially affect the implant-tissue interface. Grafts are likely to be exposed to higher shearing forces when not covered by conjunctiva completely, which may result in graft breakdown before integration. Although we used suture tarsorrhaphies to counteract the effect of blinking during the crucial early stages of fibrovascular ingrowth, it may have been insufficient for eyelid stabilization. These studies yet again confirm that the failure of epithelial and fibroblastic elements to migrate over and cover exposed areas of e-PTFE graft material will invariably result in graft extrusion.15
Although the current experimental study could not prove that e-PTFE covered with amniotic membrane is superior to bare e-PTFE with respect to graft extrusion, using a rabbit model, we found excellent incorporation and soft tissue tolerance for the e-PTFE implant when entirely covered with a layer of conjunctival epithelium. The e-PTFE graft, which is not reabsorbed and provides excellent rigidity, may improve predictability and the long-term results of lower eyelid retraction correction. The main disadvantage of this method is the risk of extrusion, which we believe may be prevented by an appropriate surgical incision and pore size modification.
Figures and Tables
Fig. 2
Dissection of lower eyelid through transconjunctival incision. (A) Conjunctiva and lower eyelid retractors were detached from the inferior tarsal border and recessed inferiorly. (B) e-PTFE sewn into place between the inferior tarsal border and recessed lower lid retractors. (C) Amniotic membrane was implanted over e-PTFE.
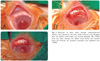
Fig. 3
Dissection of lower eyelid through subciliary cutaneous incision. (A) Lower eyelid retractors were detached from the inferior tarsal border and recessed inferiorly. (B) Several interrupted 6-0 polygalactin sutures were used to secure the e-PTFE to the inferior tarsal margin.

References
1. Tanenbaum M, McCord CD Jr, Nunery WR. McCord CD, Tanenbaum M, Nunery WR, editors. Graves' Ophthalmopathy. Oculoplastic Surgery. 1995. 3rd ed. New York: Raven Press;chap. 14.
2. Cohen MM, Lessell S. Retraction of the lower eyelid. Neurology. 1979. 29:386–389.
3. Chang EL, Rubin PAD. Upper and lower eyelid retraction. Int Ophthalmol Clin. 2002. 42:45–59.
4. Dryden RM, Soll DB. The use of scleral tranplantation in cicatrical entropion and eyelid retraction. Trans Am Acad Ophthalmol Otolaryngol. 1977. 83:669–676.
5. Doxanas MT, Dryden RM. The use of sclera in the treatment of dysthyroid eyelid retraction. Ophthalmology. 1981. 88:887–894.
6. Mourits MP, Koorneef L. Lid lengthening by sclera interposition for eyelid retraction in Graves' ophthalmopathy. Br J Ophthalmol. 1991. 75:344–347.
7. Baylis HI, Perman KI, Fett DR, Sutcliffe RT. Autogenous auricular cartilage grafting for lower eyelid retraction. Ophthalmic Plast Reconstr Surg. 1985. 1:23–27.
8. Wearne MJ, Sandy C, Rose GE, et al. Autogenous hard palate mucosa: the ideal lower eyelid spacer? Br J Ophthalmol. 2001. 85:1183–1187.
9. Siegel RJ. Palatal grafts for eyelid reconstruction. Plast Reconstruct Surg. 1985. 76:411–416.
10. Karesh JW, Fabrega MA, Rodrigues MM, Glaros DS. Polytetrafluoroethylene as an interpositional graft material for the correction of lower eyelid retraction. Ophthalmology. 1989. 96:419–423.
11. Levin PS, Dutton JJ. Polytef (Polytetrafluoroethylene) alloplastic grafting as a substitute for mucous membrane. Arch Ophthalmol. 1990. 108:282–285.
12. Karesh JW. Biomaterials in ophthalmic plastic and reconstructive surgery. Curr Opin Ophthalmol. 1998. 9:66–74.
13. Wang J, Fan J, Nordstrom REA. Evaluation of lip augmentation with Gore-tex facial implant. Aesth Plast Surg. 1997. 21:433–436.
14. Wilson AG, Plessas SJ, Gray T, Forster IW. Lymphadenopathy after Gore-tex anterior cruciate ligament reconstruction. Am J Sports Med. 1998. 26:133–135.
15. Karesh JW, Fabrega MA, Rodrigues MM. Interpositional polytetrafluoroethylene grafts Conjunctival biocompatibility. Ophthalmic Plast Reconstr Surg. 1991. 7:278–283.
16. Cas RD, Maus M, Bilyk J, et al. Gore-tex as an orbitalimplant material. Ophthal Plast Reconstr Surg. 1998. 14:425–431.
17. Waller RR. Lower eyelid retraction: management. Ophthalmic Surg. 1978. 9:41–47.
18. Holds JB, Anderson RL, Thiese SM. Lower eyelid retraction: a minimal incision surgical approach to retractor lysis. Ophthalmic Surg. 1990. 21:767–771.
19. Karesh JW. Polytetrafluoroethylene as a graft material in ophthalmic plastic and reconstructive surgery: an experimental and clinical study. Ophthalmic Plast Reconstr Surg. 1987. 3:179–185.
20. Fabrega MA, Glaros DS, Karesh JW. Biocompatibility of polytetrafluoroethylene grafts: in vitro and in vivo studies. ARVO Abstracts. Invest Ophthalmol Vis Sci. 1988. 29:S212.
21. Hanel KC, McCabe C, Abbott WM, et al. Current PTFE grafts: a biomechanical, scanning electron, and light microscopic evaluation. Ann Surg. 1982. 195:456–463.
22. Choo PH, Carter SR, Crawford JB, Seiff SR. Exposure of expanded polytetrafluoroethylene-wrapped hydroxyapatite orbital implant: A report of two patients. Ophthalmic Plast Reconstr Surg. 1998. 15:77–78.
23. Kim JC, Tseng SCG. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.
24. Lee SH, Tseng SCG. Amniotic membrane transplantation for persistent epithelial defects with ulceration. Am J Ophthalmol. 1997. 123:303–312.
25. Prabhasawat P, Barton K, Burkett G, Tseng SCG. Comparison of conjunctival autografts, amniotic membrane grafts, and primary closure for pterygium excision. Ophthalmology. 1997. 104:974–985.
26. Tseng SCG, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997. 124:765–774.
27. Ti SE, Tow SLC, Chee SP. Amniotic membrane transplantation in entropion surgery. Ophthalmology. 2001. 108:1209–1217.
28. Morton AD, Nelson C, Ikada Y, Elner VM. Porous polyethylene as a spacer graft in the treatment of lower eyelid retraction. Ophthalmic Plast Reconstr Surg. 2000. 16:146–155.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download