Abstract
Purpose
This study aimed to determine the relationship between the heat shock protein 70 from hsps70.1 and 70.3 on retinal photic injury after systemic hyperthermia.
Methods
Eight-week-old female C57BL/6 mice were kept at a constant temperature of 41~42℃ for 25~30 minutes. After dark-adaptation for 8 hours, intense light of 11000 lux was maintained for 6 hours. Histology and immunohistochemistry for the inducible heat shock protein 70 (hsp70), the constitutive heat shock protein 70 (hsc70), and western blot analysis, reverse transcriptase-polymerase chain reaction for hsp70.1 and hsp70.3 were performed just before photic injury and after 1, 4, 7, and 14 days.
Results
Light-induced retinal degeneration was prevented by thermotolerance. After hyperthermia, hsp70 was densely expressed in the inner segment of the photoreceptor layer on the photic injury. Hsp70 expression increased for 4 days after photic injury and slowly decreased thereafter. mRNA from hsp70.3 was induced earlier than that of hsp70.1.
Conclusions
Retinal photic injury was prevented by hyperthermia-induced hsp70. Hsp70 from hsp70.3 may be a rapid and short-lived responder, and that from hsp70.1 is a slower and more sustained responder. Hsp70 from hsp70.3 may be an initial retinal chaperone while hsp70 from hsp70.1 may be a sustained chaperone.
Go to : 
REFERENCES
2. Hightower LE. Heat shock, stress protein, chaperone, and proteotoxicity. Cell. 1991; 66:191–7.
4. Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992; 72:1063–81.

5. Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998; 16:833–8.

6. Rassow J, Von Ahsen O, Boemer U, Pfanner N. Molecular chaperones: towards a characterization of the heat-shock protein 70 family. Trends Cell Biol. 1997; 7:129–33.
7. Beckmann RP, Mizzen LA, Welch WJ. Interaction of HSP 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990; 248:850–4.

8. Yufu Y, Nishimura J, Ideguchi H, Nawata H. Enhanced synthesis of heat shock proteins and augmented thermotolerance after induction of differentiation in HL-60 human leukemia cells. FEBS Lett. 1990; 268:173–6.

9. Lee YJ, Hou Z-Z, Curetty L, Corry PM. Expression, synthesis, and phosphorylation of HSP 28 family during development and decay thermotolerance in CHO plateau-phase cells. J Cell Physiol. 1992; 150:441–6.
10. Yokota S, Kitahara M, Nagata K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000; 60:2942–8.
11. Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988; 30:1817–20.

12. Landry J, Bernier D, Chretien P, et al. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982; 42:2457–61.
13. Tytell M, Barbe MF, Brown IR. Induction of heat shock (stress) protein 70 and its m RNA in the normal and light-damaged rat retina after whole body hyperthermia. J Neurosci Res. 1994; 38:19–31.
14. Dean DO, Kent CR, Tytell M. Constitutive and inducible heat shock protein 70 immunoreactivity in the normal rat eye. Invest Ophthalmol Vis Sci. 1999; 40:2952–62.
15. Kim JH, Yu YS, Kim JH, et al. Immunoreactivity of constitutive and inducible heat shock protein 70 in human fetal retina. Korean J Ophthalmol. 2003; 17:14–8.

16. Yu YS, Heo JH, Hwang SW, et al. Effect of the absence of heat shock protein 70.1 (hsp70.1) on retinal photoreceptors in normal and rd mice. Korean J Ophthalmol. 2001; 15:67–73.
17. Kim JH, Yu YS, Chung H, et al. Effect of the absence of heat shock protein 70.1 (hsp70.1) on retinal photic injury. Korean J Ophthalmol. 2003; 17:7–13.

18. Lewden O, Garcher C, Assem M, et al. Changes of the inducible heat shock protein70 mRNA level in rat retina after ischemia and reperfusion. Ophthalmic Res. 1998; 30:291–4.
19. Milner CM, Campbell RD. Structure and expression of the three MHC- linked hsp70 genes. Immunogenetics. 1990; 32:242–51.

20. Hunt C, Calderwood S. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Gene. 1990; 87:199–204.

21. Dix DJ, Garges JB, Hong RL. Inhibition of hsp70-1 and hsp70-3 expression disrupts preimplantation embryogenesis and heightens embryo sensitivity to Arsenic. Mol Reprod Dev. 1998; 51:373–80.
22. Nakagawa T, Okano H, Furuichi T, et al. Novel subtypes of the mouse inositol 1,4,5-trisphosphate receptor are expressed in tissue- and developmentally specific manner. Proc Natl Acad Sci USA. 1991; 88:6244–8.
23. Udono H, Srivastava PK. Heat shock protein 70 associated peptides elicit specific cancer immunity. J Exp Med. 1993; 178:1391–6.
24. Tower J. Trasgenic method for increasing Dorsophilia life span. Mech Ageing Dev. 2000; 118:1–14.
25. Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001; 36:341–52.

26. Minois N, Khazaeli AA, Curtsinger JW. Locomotor activity as a function of age and life span in Drosophilia melanogaster overexpression hsp70. Exp Gerontol. 2001; 36:1137–53.
27. Ding XZ, Tsokos GC, Kiang JG. Overexpression of hsp70 inhibits the phosphrylation of HSF1 by activating protein phosphatase and inhibiting protein kinase C activity. FASEB J. 1998; 12:451–9.
28. Liossis SNC, Ding XZ, Kiang JG, Tsokos GC. Over-expression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat cells. J Immunol. 1997; 158:5668–75.
Go to : 
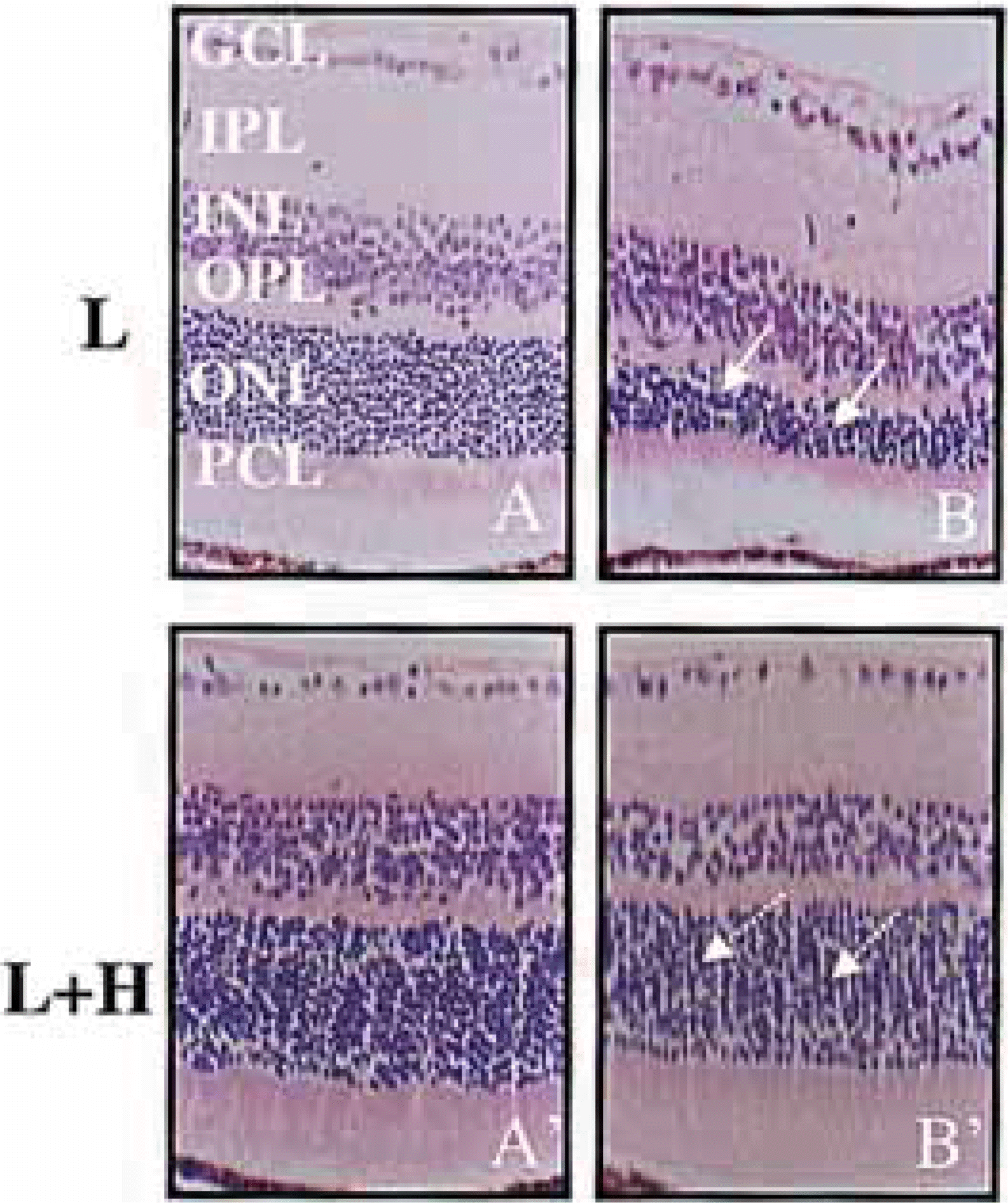 | Fig. 1.Histology of the retina after photic injury with or without hyperthermia. On photic injury without previous hyperthermia, diffuse disarrangements in the ONL and photoreceptor cell layer was observed (white arrows in B). However, photic injury after hyperthermia made no definite change in retina structure (white arrows with dotted line in B’). Hematoxylin & Eosin staining, magnification ×400 (A, A’: before photic injury; B, B’: 14 days after photic injury, L; light stress only, L+H; light stress after systemic hyperthermia) |
 | Fig. 2.Immunohistochemistry for hsp70 and hsc70 in the retina. Hsp70 expression was most prominent in the inner segment of photoreceptors (black arrows) for 4 days after photic injury following hyperthermia. Hsc70 expression was detected in most retinal layers (black arrows with dotted line), and nearly constant without temporal or spatial difference. Hematoxylin & Eosin staining, magnification × 400 (A, A’: before photic injury; B, B’: 1 day after photic injury; C, C’: 4 days; D, D’: 7 days; E, E’: 14 days). |
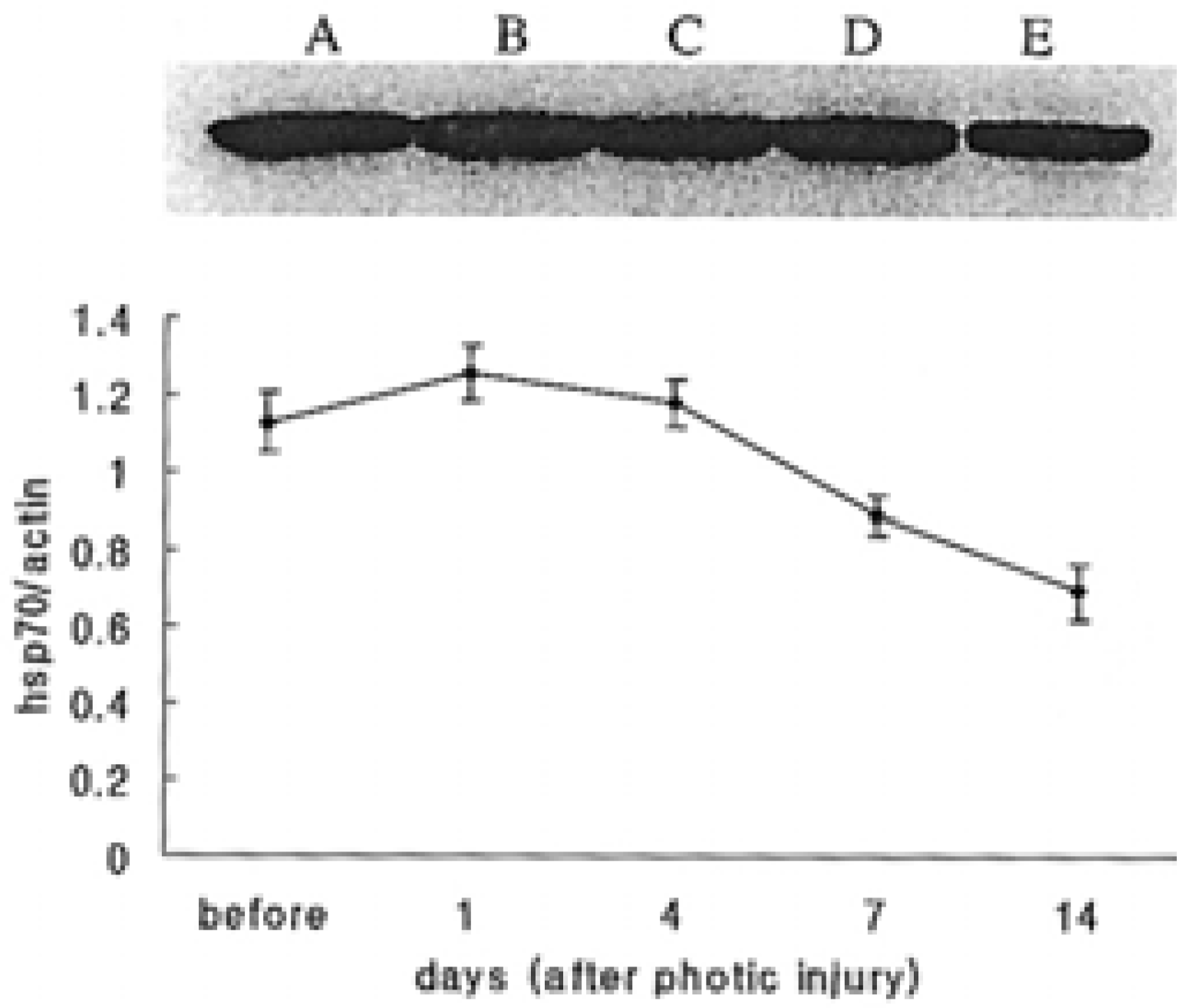 | Fig. 3.Western blot analysis for hsp70. In A, enhanced hsp70 was due to hsp induction by systemic hyperthermia. The expression of hsp70 was sustained for 7 days. Although changes were subtle, the quantitative measurement showed that production of hsp70 increased for 4 days after photic injury and slowly decreased thereafter. (A: before photic injury; B, C, D and E: 1, 4, 7 and 14 days after photic injury, respectively.) |
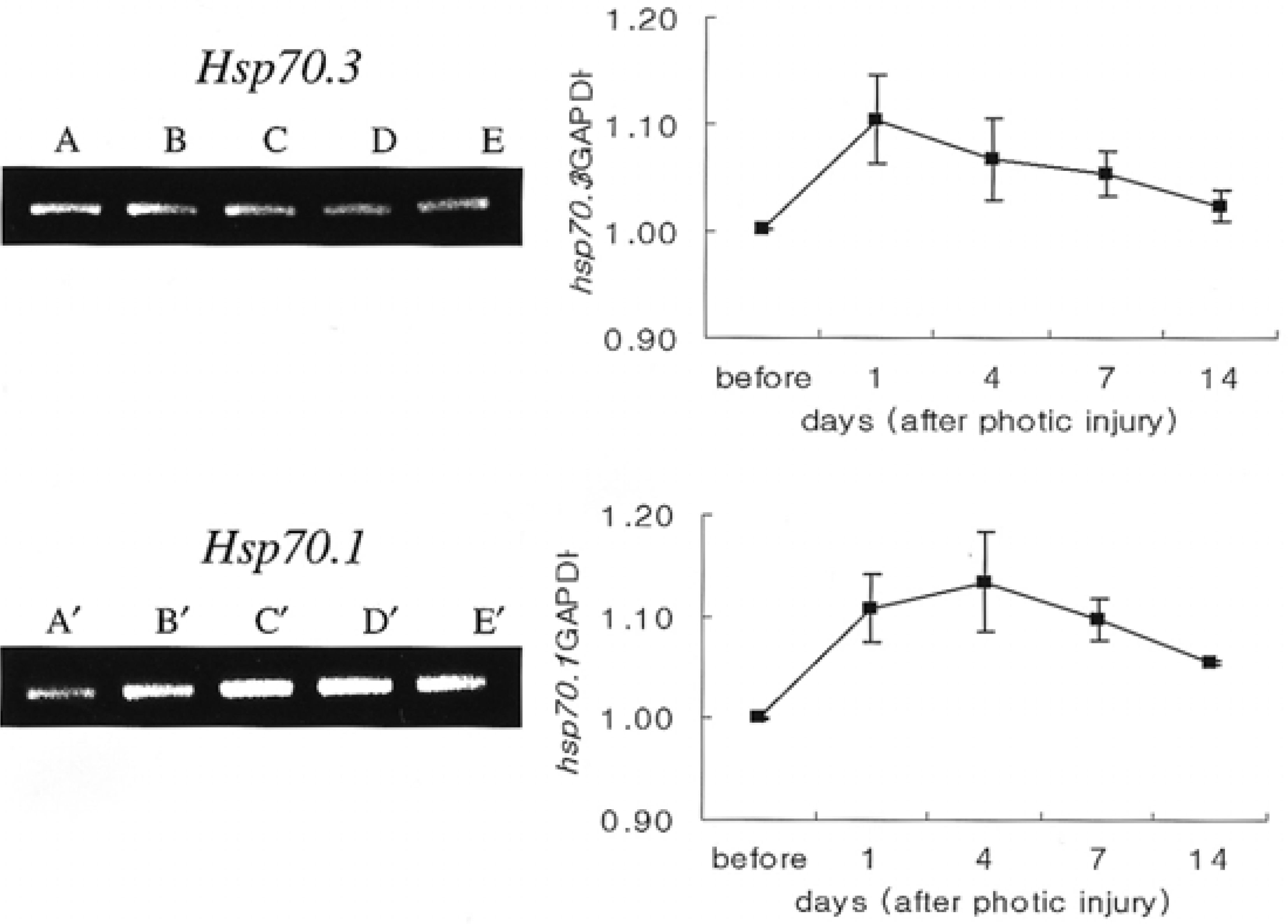 | Fig. 4.Semiquantitative analysis of mRNA expression, RT-PCR, for hsp70.1 and hsp70.3. mRNA expression of hsp70.1 reached a peak 4 days after light stress and slowly decreased until day 14. The mRNA expression in hsp70.3 was most prominent at day 1 and slowly decreased thereafter. (A, A’: before photic injury; B, B’: 1 day after photic injury; C, C’: 4 days; D, D’: 7 days; E, E’: 14 days) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download