Abstract
Neovascularization at the disc (NVD) is the most serious complication in diabetic retinopathy, and leads to vitreous hemorrhage and tractional retinal detachment. We report two cases of spontaneous regression of NVD in proliferative diabetic retinopathy. Two men (31 and 46 years old) with diabetes had NVD in both eyes. They were treated with panretinal photocoagulation on the left eye first, but their right eyes went untreated, because they did not revisit our clinic for several months. Fortunately, on revisit, their neovascularization had disappeared a few months later in both eyes, including their untreated right eyes. We could not find any specific causes for the spontaneous regression of the new vessels.
Neovascularization can occur at the disc, retina and iris of the diabetic eye.1-3 It is the most serious complication of diabetic retinopathy as it frequently leads to vitreous hemorrhage and tractional retinal detachment.4-6 It can also cause loss of visual function.
The nonperfused retina releases angiogenic factors and stimulates the growth of new vessels.1 Ablation of the nonperfused retina, by panretinal photocoagulation, usually induces regression of the new vessels.7 To the best of our knowledge, spontaneous regression of the new vessels in diabetic retinopathy has been previous reported in only three cases. We report two cases of proliferative diabetic retinopathy, in which new vessels regressed spontaneously without photocoagulative treatment.
A 31-year-old man was admitted with blurred vision in his left eye. He had had diabetes mellitus and was controlled with oral hypoglycemic agent. The ophthalmologic examination showed best corrected visual acuity of 20/20 on the right and 20/32 on the left. The anterior segment was normal in both eyes. Funduscopy revealed bilateral neovascularization of the disc (NVD) and elsewhere (NVE), microaneurysms, dot shaped retinal hemorrhages, hard exudates, cotton wool spots, intraretinal microvascular abnormalities and venous beadings (Fig. 1A). Fluorescein angiography showed a capillary retinal nonperfusion area and bilateral hyperfluorescence of the new vessels (Fig. 1B). Three quadrants in the right eye and two in the left eye of capillary nonperfusion were detected. Panretinal photocoagulation was done three times on the left eye. However, thereafter he did not visit our clinic for 9 months, at which point he returned due to blurred vision on his right eye. The best corrected visual acuity was 20/32 on the right and 20/25 on the left. Slit-lamp biomicroscopy showed three positive cell reactions in the anterior chamber on the right eye. Vitreous hemorrhages were reabsorbed in the left eye. The NVD and capillary nonperfusion areas had disappeared in both eyes in funduscopy and angiography (Fig. 2A,B). After one-month treatment with steroid eye drops, the anterior uveitis was healed. One year later, there was no neovascularization in either eye. The best corrected visual acuity was 20/20 on the right and 20/25 on the left.
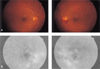
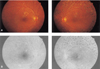
A 46-year-old man was referred with proliferative diabetic retinopathy in his left eye. He had had diabetes mellitus which had been controlled with oral hypoglycemic agent for 4 years. The ophthalmologic examination showed best corrected visual acuity of 20/32 on the right and 20/50 on the left. The anterior segment was normal in both eyes. Funduscopy revealed bilateral NVD, microaneurysms, dot shaped retinal hemorrhages, intraretinal microvascular abnormalities and preretinal hemorrhage in the left eye (Fig. 3A). Fluorescein angiography showed bilateral hyperfluorescence of the new vessels and multiple dot shaped hyperfluorescence (Fig. 3B). Panretinal photocoagulation was done on his left eye. Two months later, he had a subhyaloid hemorrhage and tractional band on macula in the left eye. Therefore we performed pars plana vitrectomy, removal of tractional band and endophotocoagulation. Three months postoperatively, best corrected visual acuity was 20/20 on the right and 20/40 on the left. The NVD areas were absent in both fundi (Fig. 4A,B). Eight months later, there was no neovascularization in either eye.
Retinal neovascularization is caused by many factors such as retinal ischemia and inflammatory stimulus. Thus neovascularization may be the end response to many, varied stimuli. Previous reports have also associated optic disc neovascularization with inflammation. It is caused by liberating a substance that diffuses back to the optic disc.2,8 A similar process occurs in other ocular tissues such as the cornea, iris and choroid.2 Neovascularization appears to be an adaptive healing mechanism used by the body in response to various tissue insults. It is a dynamic process that requires a continuing stimulus, and will usually regress with the cessation of the stimulus.1
The optic disc appears to be more susceptible to developing neovascularization than is the peripheral retina.2,18-20 This may be related to the thinness of and occasional gaps in the internal limiting membrane in this area,21 drainage of vasoproliferative factors through the vitreopapillary pathway, resulting in increased exposure of this area,2 involvement of the ciliary circulation in producing NVD,22 or a combination of these factors.
Diabetic patients with NVD have a poor visual prognosis, and there is a high incidence of vitreous hemorrhage as well as fibrous proliferation and traction retinal detachment.4 In diabetes, blood-retinal barrier breakdown occurs due to retinal ischemia, vascular change and hemorrhage.9-11 The release of angiogenic factors develops new vessels.1 Conventionally, panretinal photocoagulation is essential to the regression of new vessels in diabetic retinopathy.
At the present level of knowledge, interpreting our cases is difficult. In both, neovascular regression occurred without destruction of the ischemic retina, which is the basis of proliferative diabetic retinopathy treatment. Neither of these patients received corticosteroids or underwent other anti-inflammatory therapy. However, they both also complained of general weakness and poor control of glucose level at first visit. So there was a possibility of temporary retinal ischemic change due to poor glucose control.
We discounted the possibility that our two patients had uveitis because of the decidedly diabetic clinical and angiographic characteristics of their lesions. We also discounted the possibilities of involutional diabetic retinopathy and diabetic papillopathy.12-17 Involutional diabetic retinopathy is characterized by changes in the appearance of arteries and veins. Veins become narrower and often appear sheathed, and fewer small branches are visible. The caliber of arterioles decreases, and there is a marked reduction in the number of visible branches. In our patients, however, there were no characteristics of involutional diabetic retinopathy. Diabetic papillopathy was excluded because of the preretinal hemorrhages very close to the vascular lesions, typical of neovascularization and atypical of papillopathy and because of the lesions in the preretinal area, far from the optic discs.
We report two cases of very unusual spontaneous regression of NVD in proliferative diabetic retinopathy.
Figures and Tables
Fig. 1
Case 1. (A) Top: With proliferative DM retinopathy, neovascularization of the optic disc is seen on both eyes. (B) Bottom: Angiography shows multiple, severe leakage from neovascularization of the disc and retina on both eyes.
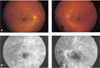
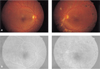
Fig. 2
Case 1. (A) Top: Neovascularization of the optic disc has disappeared on both eyes. (B) Bottom: Angiography shows no active leakage at the disc on either eyes.
References
1. Bandello F, Gass JD, Lattanzio R, Brancato R. Spontaneous regression of neovascularization at the disk and elsewhere in diabetic retinopathy. Am J Ophthalmol. 1996. 122:494–501.
2. Henkind P. Ocular neovascularization. Am J Ophthalmol. 1978. 85:287–301.
3. Patz A. Clinical and experimental studies on retinal neovascularization. Am J Ophthalmol. 1982. 94:715–743.
4. Yaval Y, Linda WP, Stuart LF, Lawrence S, David HO, Arnall P. Optic disc neovascularisation in diabetic retinopathy: II. Natural history and results of photocoagulation treatment. Br J Ophthalmol. 1980. 64:77–86.
5. Taylor E, Dobree JH. Proliferative diabetic retinopathy: site and size of initial lesions. Br J Ophthalmol. 1970. 54:11–23.
6. Little HL. Argon laser photocoagulation of proliferative diabetic retinopathy. Int Ophthalmol Clin. 1976. 16:79–103.
7. Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy: the second report of Diabetic Retinopathy Study findings. Ophthalmology. 1978. 85:82–106.
8. Shorb SR, Irvine AR, Kimura SJ, Morris BW. Optic disk neovascularization associated with chronic uveitis. Am J Ophthalmol. 1976. 82:175–182.
9. Schatz H, Patz A. Cystoid maculopathy in diabetics. Arch Ophthalmol. 1976. 94:761–768.
10. Kearns M, Hamilton AM, Kohner EM. Excessive permeability in diabetic maculopathy. Br J Ophthalmol. 1979. 63:489–497.
11. Bonnet M, Bensoussan B, Grange JD, Pingault C, Francoz N. Capillaropathie oedemateuse aigue du diabetique insulin-dependant. J Fr Ophtalmol. 1982. 5:303–316.
12. Beetham WP. Visual prognosis of proliferative diabetic retinopathy. Br J Ophthalmol. 1963. 47:611–619.
13. Davis MD. Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol. 1965. 74:741–751.
14. Dobree JH. Proliferative diabetic retinopathy: evolution of the retinal lesions. Br J Ophthalmol. 1964. 48:637–649.
15. Ramsay WJ, Ramsay RC, Purple RL, Knoblock WH. Involutional diabetic retinopathy. Am J Ophthalmol. 1977. 84:851–858.
16. Davis MD. Ryan SJ, editor. Proliferative diabetic retinopathy. Retina. 1989. St. Louis: CV Mosby Co.;367–402.
17. Brancato R, Menchini U, Brndello F. Diabetic papillopathy: fluoroangiographic aspects. Metab Pediatr Syst Ophthalmol. 1986. 9:57–61.
18. Peter JK, John JW. Resolution of optic disk neovascularization associated with intraocular inflammation. Am J Ophthalmol. 1980. 90:545–548.
19. Shabo AL, Maxwell DS. Experimental immunogenic proliferative retinopathy in monkeys. Am J Ophthalmol. 1977. 83:471–480.
20. Brucker AJ. Disk and peripheral retina neovascularization secondary to talc and cornstarch emboli. Am J Ophthalmol. 1979. 88:864–868.
21. Foos RY. Vitreoretinal juncture, topographical variations. Invest Ophthalmol. 1972. 11:801–806.
22. Asdourian GK, Goldberg MF, Busse B. Optic disk neovascularization of uveal origin. Arch Ophthalmol. 1977. 95:998–1002.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download