Abstract
The aim was to investigate the proteolytic activity of plasmin and its long-term complications. Plasmin was injected into the vitreous cavity of rabbits' eyes. Slit lamp biomicroscopy and electroretinography were performed. Rabbits were serially sacrificed at four months, and globes fixated and prepared for light and transmission electron microscopy. In both the plasmin-injected and control eyes, electroretinography showed a transient decrease in the amplitude, but this recovered to the baseline level in a week. Under the light microscope, the plasmin-treated eyes had a smooth retinal surface, implying separation of the vitreous cortex from the retina. In the control eyes, the collagen fibers remained on the retinal surface. By transmission electron microscopy, the plasmin-treated eyes showed a vitreous-free retinal surface, but no vitreoretinal separation was observed in the control eyes. Plasmin induces a cleavage between the vitreous and the internal limiting membrane, with no long-term complications, so may be a useful pharmacologic adjunct to vitrectomy.
The relief of vitreoretinal traction is one of the prime purposes of vitrectomy. Despite the much improved surgical techniques and equipment, the complete separation of the vitreous cortex from the internal limiting membrane (ILM) remains difficult. Moreover, the mechanical peeling of the hyaloid from the retinal surface may be associated with the risk of iatrogenic retinal breaks and hemorrhages.1 For some time, vitreoretinal surgeons have requested minimally invasive vitreolytic solutions for surgical problems in cases of vitreoretinal disease, such as diabetic retinopathy and macular hole. Many efforts have been made to develop chemicals that cause selective vitreolysis, especially in children and young adults in whom the adhesion between the hyaloid and the internal limiting membrane is tight.
A number of vitreolytic substances have been investigated, including hyaluronidase, dispase, tissue plasminogen activator and plasmin. Plasmin is a nonspecific protease, which acts upon laminin and fibronectin, components of the vitreoretinal interface. It has been reported that an intravitreal injection of plasmin facilitates posterior vitreous detachment (PVD), with or without an additional procedure.2-4 Gandorfer and colleagues5 demonstrated that plasmin induced a cleavage between the vitreous cortex and the ILM, producing a smooth retinal surface, without additional surgery, in experimental pig eyes. Verstraeten and colleagues4 found that the ERGs in plasmin injected eyes were decreased in amplitude one 1 hour after the injection, and that this was followed by the gradual recovery over several days. However, there has been no previous report on the long-term effects, or of the toxicity, of plasmin on the retina.
In this study, the aim was to investigate the proteolytic activity of plasmin on the vitreoretinal interface, and identify any long-term complications.
A stock solution of plasmin was prepared from lyophilized plasmin powder (EC 3.4.21.7, Sigma-Aldrich, Germany) in calcium- and magnesium-free phosphate buffered saline (PBS), diluted to a final concentration of 0.5 U/0.1 ml and stored at -20℃ until required.
Twelve young New Zealand white rabbits (2-2.5kg) were housed in the animal care facilities of Seoul National University Hospital Clinical Research Institute. Rabbits were anesthetized with a combination of an intramuscular injection of ketamine hydrochloride and xylazine hydrochloride. One eye of each rabbit underwent pars plana injection of 0.5 U of porcine plasmin, 2mm from the limbus into the midvitreous cavity, using a 30 G needle. The fellow eye received a 0.1 ml injection of PBS, which acted as a control.
The rabbits were anesthetized, and examined daily, using slit lamp biomicroscopy and an indirect ophthalmoscope, until day 7, and followed once a month for 4 months.
All 12 eyes underwent electroretinography on the 1st, 3rd and 7th days, and after the rabbits were sacrificed, the remaining eyes underwent electroretinography on the 1st, 2nd and 4th months. The rabbits were anesthetized with a combined intramuscular injection of ketamine and xylazine. The pupils were dilated, and topical proparacaine applied to the cornea. The rabbits were dark-adapted for 1hour before the recording commenced. Electrodes were then placed, with the contact electrode on the cornea, the needle electrode in the skin of glabella, and the ground electrode into the skin of the ear. For recording, a flash stimulator, a biophysical amplifier and averager were used (Neuropack II, Nihon Kohden, Japan). Dark-adapted ERGs were recorded for a light stimulus of 1.2J.
12 rabbits were serially sacrificed, and later three rabbits each were sacrificed at 1-week and 1-, 2- and 4-months. Bilateral enucleation was performed. Three pars plana sclerotomies were made with a #11 mess to ensure rapid penetration of the fixative. Globes were soaked in cups filled with 2% paraformaldehyde and 2.5% glutaraldehyde. After 24 hour of fixation, the globes were hemisected, and small sections prepared for transmission electron microscopy. One hemi-globe was also processed for light microscopy.
Specimens for the transmission electron microscopy were postfixed in 1% osmium tetroxide, dehydrated in increasing grades of ethanol, substituted with propylene oxide, and then embedded in epoxy resin (Epon 812). Semithin sections were stained with 1% toluidine blue, and ultrathin sections contrasted with uranyl acetate and lead citrate, and then photographed using a model 7100 electron microscope (Hitachi, Japan).
Both the plasmin- and PBS-treated eyes showed flare in the anterior chamber and vitreous haziness on the 1st day. In the PBS-treated eyes, the flare had cleared by the 3rd day, showing a clear anterior chamber, clear vitreous and flat fundus, whereas the plasmin-treated eyes still showed flare and vitreous haziness. However, on the 7th day, the anterior chamber and vitreous were clear in all eyes, showing a flat fundus, without morphologic abnormality, which were maintained throughout the 4 months of examination.
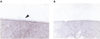
The PBS-injected control eyes, sacrificed at 2 months, showed irregular retinal surfaces, with adherent vitreous (Fig. 2-A). The plasmin-injected eyes, sacrificed at 2 months, demonstrated clear, smooth retinal surfaces (Fig. 2-B). These findings were first observed on the eyes sacrificed at 1 week, and were maintained for the 4 months of the experiment. No retinal necrosis or abnormalities of the retinal morphology or cellular anatomy were observed in either the plasmin-injected or control eyes.
The transmission electron micrographs of the control eyes, sacrificed at 2 months, showed persistent vitreous attachment, with the collagen fibers condensed over the retinal surface (Fig. 3-A). In the plasmin-treated eyes, sacrificed at 2months, collagen fibers could be rarely seen adjacent to the ILM in some areas (Fig. 3-B). These findings were first observed in the eyes sacrificed on 1 week, and were maintained for the 4 months of the experiment. All the retinal layers, including the photoreceptor outer segments and retinal pigment epithelial cells, showed a normal appearance, irrespective of the plasmin-treatment, during the follow-up period.
It has been reported that an intravitreal plasmin injection, when used alone, or in combination with vitrectomy or an intravitreal gas injection, facilitates cleavage between the vitreous cortex and the ILM.3-5 Margherio and his colleagues6 used autologous plasmin, in pediatric patients to induce PVD prior to a vitrectomy for traumatic macular holes, and demonstrated the successful closure of the macular holes, with significant visual improvement. Thus, it appeared that autologous plasmin, used as an adjunct to vitrectomy, could facilitate the removal of the cortical vitreous from the retinal surface. However, the previous reports were limited by the short follow up periods; ca one week.
This study was designed to investigate the long-term effects, and toxicity, of plasmin on the retinal function. In this study, an intravitreal plasmin injection caused a transient decrease in the retinal function, represented by a reduced ERG amplitude exceeding 50%, which also occurred in all the PBS-injected eyes. Therefore, this decrease in the ERG amplitude may have been due to the intravitreal injection itself, rather than the plasmin. However, the ERG amplitude recovered fully, within a week, and did not subsequently change during the follow up period, indicating that plasmin had no long-term toxic effect on the retina. The histological examination also revealed no evidence of retinal damage in any of the plasmin treated eyes, even after the 4 month follow up.
The ILM contains types I and IV collagen, laminin, fibronectin and other glycoconjugates, which are supposed to bridge and bind the collagen fibers between the posterior vitreous cortex and the ILM.7 Moreover, plasmin is a nonspecific protease, which is known to have proteolytic activity against these components of the ILM.8,9 In the past, several enzymes have been used to induce PVD. Hyaluronidase, collagenase, chondroitinase, dispase, plasmin and tissue plasmin activator (t-PA) have been tested in animals, but only plasmin and t-PA have been examined in humans.10-12 Plasmin acts immediately after injection, whereas t-PA needs some hours to degenerate the plasmin from its precursor, plasminogen, which gives plasmin a significant advantage, and allows it to be administered at the beginning of a pars plana vitrectomy.
Significantly, previous studies have been performed just over a short periods,4,5 which investigated the intraocular effects of plasmin for just a week. In the present study, the plasmin-injected rabbit eyes were followed for 4 months, and found to show posterior vitreous detachment, with no significant toxicity observed.
This study shows that plasmin alone, injected into the vitreous cavity, can separate the vitreal cortex from the retinal surface, with no significant longterm toxic effects on the retinal structure or function. These results suggest that plasmin may prove to be a useful and safe biochemical adjunct to a mechanical vitrectomy.
Figures and Tables
Fig. 1
A: Electroretinograms of a control eye (PBS injection). B: Electroretinograms of a plasmin-treated eye. The PBS- and plasmin-injected eyes show decreased amplitude in the ERGs recorded on days 1 and 3. However by the 7th day post injection, the amplitude of the ERGs has recovered, with no difference from that of the baseline ERGs. These findings were maintained 4 months after the injection.

Fig. 2
A: light micrograph of a control eye 2 months after a PBS injection. Note the irregular, ragged retinal surface (PAS, ×400). B: light micrograph of a plasmin-treated eye 2 months post injection. Note the clear, smooth retinal surface (PAS, ×400).

Fig. 3
A: Transmission electron micrograph of a control eye 2 months after a PBS injection. Note the persistent cortical vitreous attached to the internal limiting membrane (arrowhead). (original magnification ×46,000). B: Transmission electron micrograph of a plasmin-treated eye 2 months post injection. Note the smooth internal limiting membrane (original magnification ×46,000).
References
1. Hutton WL, Fuller DG, Snyder WB, Fellman RL, Swanson WH. Visual field defects after macular hole surgery. A new finding. Ophthalmology. 1996. 103:2152–2158.
2. Kang SW, Hyung SM, Choi MY, Lee J. Induction of vitreolysis and detachment with hyaluronidase and perfluoropropane gas. Korean J Ophthalmol. 1995. 9:69–78.
3. Hikichi T, Yanagiya N, Kado M. Posterior vitreous detachment induced by injection of plasmin and sulfur hexafluoride in the rabbit vitreous. Retina. 1999. 19:55–58.
4. Verstraeten TC, Chapman C, Hartxer M, Winkler BS, Trese MT, Williams GA. Pharmacologic induction of posterior vitreous detachment in the rabbit. Arch Ophthalmol. 1993. 111:849–854.
5. Gandorfer A, Putz E, Welge-Lussen U, Gruterich M, Ulbig M, Kampik A. Ultrastructure of the vitreoretinal interface following plasmin assisted vitrectomy. Br J Ophthalmol. 2001. 85:6–10.
6. Margherio AR, Margherio RR, Hartzer M, Trese MT, Williams GA, Ferrone PJ. Plasmin enzyme-assisted vitrectomy in traumatic pediatric macular hole. Ophthalmology. 1998. 105:1617–1620.
7. Jerdan JA, Kao L, Glaser BM. The inner limiting membrane: a modified basement membrane? Invest Ophthalmol Vis Sci. 1986. 27:Suppl. 230.
8. Liotta LA, Goldfarb RH, Brundage R. Effect of plasminogen activator(urokinase), plasmin, and thrombin on glycoprotein and collagenous components of basement membrane. Cancer Res. 1981. 41:4629–4636.
9. Papp B, Kovacs T, Lerant I. Conditions of formation of the heparin-fibronectin-collagen complex and the effect of plasmin. Biochem Biophys Acta. 1987. 925:241–247.
10. Trese MT, Williams GA, Hartzer MK. A new approach to stage 3 macular hole. Ophthalmology. 2000. 107:1607–1611.
11. Handwerger BA, Blodi BA, Chandra SR, Olsen TW, Stevens TS. Treatment of submacular hemorrhage with low-dose intravitreal tissue plasminogen activator injection and pneumatic displacement. Arch Ophthalmol. 2001. 119:28–32.
12. Williams JG, Trese MT, Williams GA, Hartzer MK. Autologous plasmin enzyme in the surgical management of diabetic retinopathy. Ophthalmology. 2001. 108:1902–1905.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download