Abstract
We evaluated the effect of a basic fibroblast growth factor (bFGF) and saporin conjugate (bFGF-SAP) on proliferation, migration and tubule formation in bovine choriocapillary endothelial cells (BCECs). Cell proliferation and MTS assays were done with 0.01, 0.1, 1, 10, and 100 nM bFGF-SAP, and an equimolar concentration of bFGF and saporin. TUNEL assay was performed to confirm apoptosis. Cells were treated with 1, 10, and 100 nM bFGF-SAP and migration assay and tubule formation assay were done. Results were evaluated with image analysis. All experiments were performed in triplicate and repeated three times. Viable cells (ID50 = 0.62) and cell proliferation by MTS assay (ID50 = 0.75 nM) were inhibited. Saporin caused cytotoxicity and inhibition of proliferation at high concentration. DNA fragmentation was identified by TUNEL assay. Migration and tubule formation were also inhibited. All mechanisms responsible for neovascularization were inhibited, and this could be applied in the management of subretinal choroidal neovascularization (SRN).
References
1. Sarks SH. New vessel formation beneath the retinal pigment epithelium in senile eye. Br J Ophthalmol. 1973; 57:951–965.
2. Teeters VW, Bird AC. A clinical study of the vascularity of senile disciform macular degeneration. Am J Ophthalmol. 1973; 75:53–65.

3. Ryan SJ, Mittl RN, Maumenee AE. The disciform response: an historical perspective. Graefe's Arch Clin Exp Ophthalmol. 1980; 215:1–20.

5. Macular Photocoagulation Study Group. Argon laser photocoagulation for neovascular maculopathy after five years. Arch Ophthalmol. 1991; 109:1109–1114.
6. Frank RN, Amin RH, Puklin JE. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am J Ophthalmol. 1999; 127:694–709.
7. Jampol LM. Antioxidants, zinc, and age-related macular degeneration: results and recommendations. Arch Ophthalmol. 2001; 119:1533–1534.
8. Jampol LM, Ferris FL 3rd. Antioxidants and zinc to prevent progression of age-related macular degeneration. JAMA. 2001; 286:2466–2468.
9. Schmidt-Erfurth U, Miller J, Sickenberg M, Bunse A, Laqua H, Gragoudas E, Zografos L, Birngruber R, van den Bergh H, Strong A, Manjuris U, Fsadni M, Lane AM, Piguet B, Bressler NM. Photodynamic therapy of subfoveal choroidal neovascularization: clinical and angiographic examples. Graefe's Arch Clin Exp Ophthalmol. 1998; 236:365–374.

10. Ladd BS, Solomon SD, Bressler NM, Bressler SB. Photodynamic therapy with verteporfin for choroidal neovascularization in patients with diabetic retinopathy. Am J Ophthalmol. 2001; 132:659–667.

11. Bressler NM. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization-verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2002; 133:168–169.

13. Beitz JG, Davol P, Clark JW, Kato J, Medina M, Frackelton AR, Lappi DA, Baird A, Calabresi P. Antitumor activity of basic fibroblast growth factor-saporin mitotoxin in vitro and vivo. Cancer Res. 1992; 52:227–230.
14. Siena S, Lappi DA, Bregni M, Formosa A, Villa S, Soria M, Bonadonna G, Gianni AM. Synthesis and characterization of an antihuman T-lymphocyte saporin immunotoxin (OKT1-SAP) with in vivo stability into nonhuman primates. Blood. 1988; 72:756–765.

15. Santanche S, Belleli A, Brunori M. The unusual stability of saporin, a candidate for the synthesis of immunotoxins. Biochem Biophys Res Commun. 1997; 234:129–132.
16. Lappi DA, Martineau D, Baird A. Biological and chemical characterization of basic FGF-saporin mitotoxin. Biochem Biophys Res Commun. 1989; 160:917–923.

17. Lappi DA, Martineau DG, Nakamura S, Buscaglia M, Baird A. Basic fibroblast growth factor-saporin mitotoxin: an endothelial cell growth inhibitor. J Cell Biochem. 1990; 14E(Suppl.):222.
18. Lappi DA, Maher PA, Martineau D, Baird A. The basic fibroblast growth factor mitotoxin acts through the basic fibroblast growth factor. J Cell Physiol. 1991; 147:17–26.
19. Axel DI, Riessen R, Runge H, Viebahn R, Karsch KR. Effects of cerivastatin on human arterial smooth muscle cell proliferation and migration in transfilter cocultures. J Cardiovasc Pharmacol. 2000; 35:619–629.

20. Lehr HA, van der Loos CM, Teeling P, Gown AM. Complete chromogen separation and analysis in double immunohistochemical stains using Photoshop-based image analysis. J Histochem Cytochem. 1999; 47:119–126.

21. Matsumura T, Wolff K, Petzelbauer P. Endothelial cell tube formation depends on cadherin 5 and CD31 interactions with filamentous actin. J Immunol. 1997; 158:3408–3416.
22. Murata T, He S, Hangai M, Ishibashi T, Xi XP, Kim S, Hsueh WA, Ryan SJ, Law RE, Hinton DR. Peroxisome proliferator-activated receptor-gamma ligands inhibit choroidal neovascularization. Invest Ophthalmol Vis Sci. 2000; 41:2309–2317.
23. Yamakawa K, Bhutto IA, Lu Z, Watanabe Y, Amemiya T. Retinal vascular changes in rats with inherited hypercholesterolemia – corrosion cast demonstration. Curr Eye Res. 2001; 22:258–265.

24. Kobayashi H, Kobayashi K. Radiotherapy for subfoveal neovascularisation associated with pathological myopia: a pilot study. Br J Ophthalmol. 2000; 84:761–766.

25. Cho J, Jun BK, Lee YJ, Uhm KB. Factors associated with the poor final visual outcome after traumatic hyphema. Korean J Ophthalmol. 1998; 12:122–129.

27. Hargrave S, Weakley D, Wilson C. Complications of ocular paintball injuries in children. J Pediatr Ophthalmol Strabismus. 2000; 37:338–343.

28. Eckstein M, Wells JA, Aylward B, Gregor Z. Surgical removal of non-age-related subfoveal choroidal neovascular membranes. Eye. 1998; 12:775–780.

29. Wolf S, Lappas A, Weinberger AW, Kirchhof B. Macular translocation for surgical management of subfoveal choroidal neovascularizations in patients with AMD: first results. Graefe's Arch Clin Exp Ophthalmol. 1999; 237:51–57.

30. Jaakkola A, Heikkonen J, Tarkkanen A, Immonen I. Visual function after strontium-90 plaque irradiation in patients with age-related subfoveal choroidal neovascularization. Acta Ophthalmol Scand. 1999; 77:57–61.

31. Flaxel CJ, Friedrichsen EJ, Smith JO, Oeinck SC, Blacharski PA, Garcia CA, Chu HH. Proton beam irradiation of subfoveal choroidal neovascularisation in age-related macular degeneration. Eye. 2000; 14:155–164.

32. Davol P, Beitz JG, Mohler M, Ying W, Cook J, Lappi DA, Frackelton AR. Saporin toxins directed to basic fibroblast growth factor receptors effectively target human ovarian teratocarcinoma in an animal model. Cancer. 1995; 76:79–85.

33. Davol P, Frackelton AR. The mitotoxin, basic fibroblast growth factor-saporin, effectively targets human prostatic carcinoma in an animal model. J Urol. 1996; 156:1174–1179.

34. Chandler LA, Sosnowski BA, McDonald JR, Price JE, Aukerman SL, Baird A, Pierce GF, Houston LL. Targeting tumor cells via EGF receptors: selective toxicity of an HBEGF-toxin fusion protein. Int J Cancer. 1998; 78:106–111.
35. Sforzini S, de Totero D, Gaggero A, Ippoliti R, Glennie MJ, Canevari S, Stein H, Ferrini S. Targeting of saporin to Hodgkin's lymphoma cells by anti-CD30 and anti-CD25 bispecific antibodies. Br J Haematol. 1998; 102:1061–1068.

36. Pastan I, Kreitman RJ. Immunotoxins for targeted cancer therapy. Adv Drug Deliv Rev. 1998; 31:53–88.
37. Davol PA, Frackelton AR. Targeting human prostatic carcinoma through basic fibroblast growth factor receptors in an animal model: characterizing and circumventing mechanisms of tumor resistance. Prostate. 1999; 40:178–191.

38. Scott CF, Lambert JM, Goldmacher VS, Blattler WA, Sobel R, Schlossman SF, Benacerraf B. The pharmacokinetics and toxicity of murine monoclonal antibodies and of gelonin conjugates of these antibodies. Int J Immunopharmacol. 1987; 9:211–225.

39. Ghetie V, Vitetta E. Immunotoxins in the therapy of cancer: from bench to clinic. Pharmac Ther. 1994; 63:209–234.

40. Bolognesi A, Tazzari PL, Olivieri F, Polito L, Falini B, Stirpe F. Induction of apoptosis by ribosome-inactivating proteins and related immunotoxins. Int J Cancer. 1996; 68:349–355.

41. Behar-Cohen FF, David T, D'Hermies F, Pouliquen YM, Buechler Y, Nova MP, Houston LL, Courtois Y. In vivo inhibition of lens regrowth by fibroblast growth factor 2-saporin. Invest Ophthalmol Vis Sci. 1995; 36:2434–2448.
42. Wee WR, Parandoosh Z, Sakamoto T, Caton M, Nova M, McDonnell PJ. Antiproliferative effect of basic fibroblast growth factor-saporin mitotoxin on keratocytes in culture. Korean J Ophthalmol. 1996; 10:1–7.

43. Bretton RH, Swearingen A, Kash RL, Cooley R. Use of a polylysine-saporin conjugate to prevent posterior capsule opacification. J Cataract Refract Surg. 1999; 25:921–929.

45. Kumar S, West DC, Ager A. Heterogeneity in endothelial cells from large vessels and microvessels. Differentiation. 1987; 36:57–70.

46. Fajardo LF. The complexity of endothelial cells: a review. Am J Clin Pathol. 1989; 92:241–250.
47. Page C, Rose M, Yacoub M, Pigott R. Antigenic heterogeneity of vascular endothelium. Am J Pathol. 1992; 141:673–683.

48. Hoffmann S, Spee C, Murata T, Cui JZ, Ryan SJ, Hinton DR. Rapid isolation of choriocapillary endothelial cells by Lycopersicon esculentum-coated Dynabeads. Graefe's Arch Clin Exp Ophthalmol. 1998; 236:779–784.
50. Goto F, Goto K, Weindel K, Folkman J. Synergistic effects of vascular endothelial growth factor and basic fibroblast growth factor on the proliferation and cord formation of bovine capillary endothelial cells within collagen gels. Lab Invest. 1993; 69:508–517.
51. Stirpe F, Bailey S, Miller SP, Bodley JW. Modification of ribosomal RNA by ribosome-inactivating proteins from plants. Nucleic Acids Res. 1988; 16:1349–1357.

52. Perkins AC, Pimm MV, Baldwin RW. Demonstration of the hepatic uptake of radiolabelled immunotoxins using gamma scintigraphy. Eur J Cancer Clin Oncol. 1987; 23:1225–1227.

53. Bergamaschi G, Cazzola M, Dezza L, Savino E, Consonni L, Lappi D. Killing of K562 cells with conjugates between human transferrin and a ribosome-inactivating protein (SO6). Br J Haematol. 1988; 68:379–384.

54. Lappi DA, Baird A. Mitotoxins: Growth factor-targeted cytotoxic molecules. Progress in Growth Factor Research. 1990; 2:223–236.

55. Bergamaschi G, Perfetti V, Tonon L, Novella A, Lucotti C, Danova M, Glennie MJ, Merlini G, Cazzola M. Saporin, a ribosome-inactivating protein used to prepare immunotoxins, induces cell death via apoptosis. Br J Haematol. 1996; 93:789–794.

56. Morse LS, Sidikaro Y. Isolation and characterization of bovine choroidal microvessel endothelium and pericytes in culture. Curr Eye Res. 1990; 9:631–642.

Fig. 1.
Characterization of bovine choriocapillary endothelial cells. Bovine choriocapillary endothelial cells showed dark brown colored positive immuno-staining for von Willebrand factor. (magnification, ×10).
Fig. 2.
Effect of bFGF-SAP, bFGF and saporin on bovine choriocapillary endothelial cells growth. A: bFGF-SAP showed a dose-dependent cytotoxicity to choriocapillary endothelial cells. Analysis by nonlinear regression using Sigmaplot V7.0 revealed a typical reverse sigmoid standard curve. ID50 was 0.62 nM. B: bFGF induced the proliferation of bovine choriocapillary endothelial cells dose-dependently. Saporin had no significant effect at lower concentrations, but showed cytotoxicity at a high concentration of 50 nM. Results are given as a mean S.D. ∗P < 0.01 compared with untreated control.
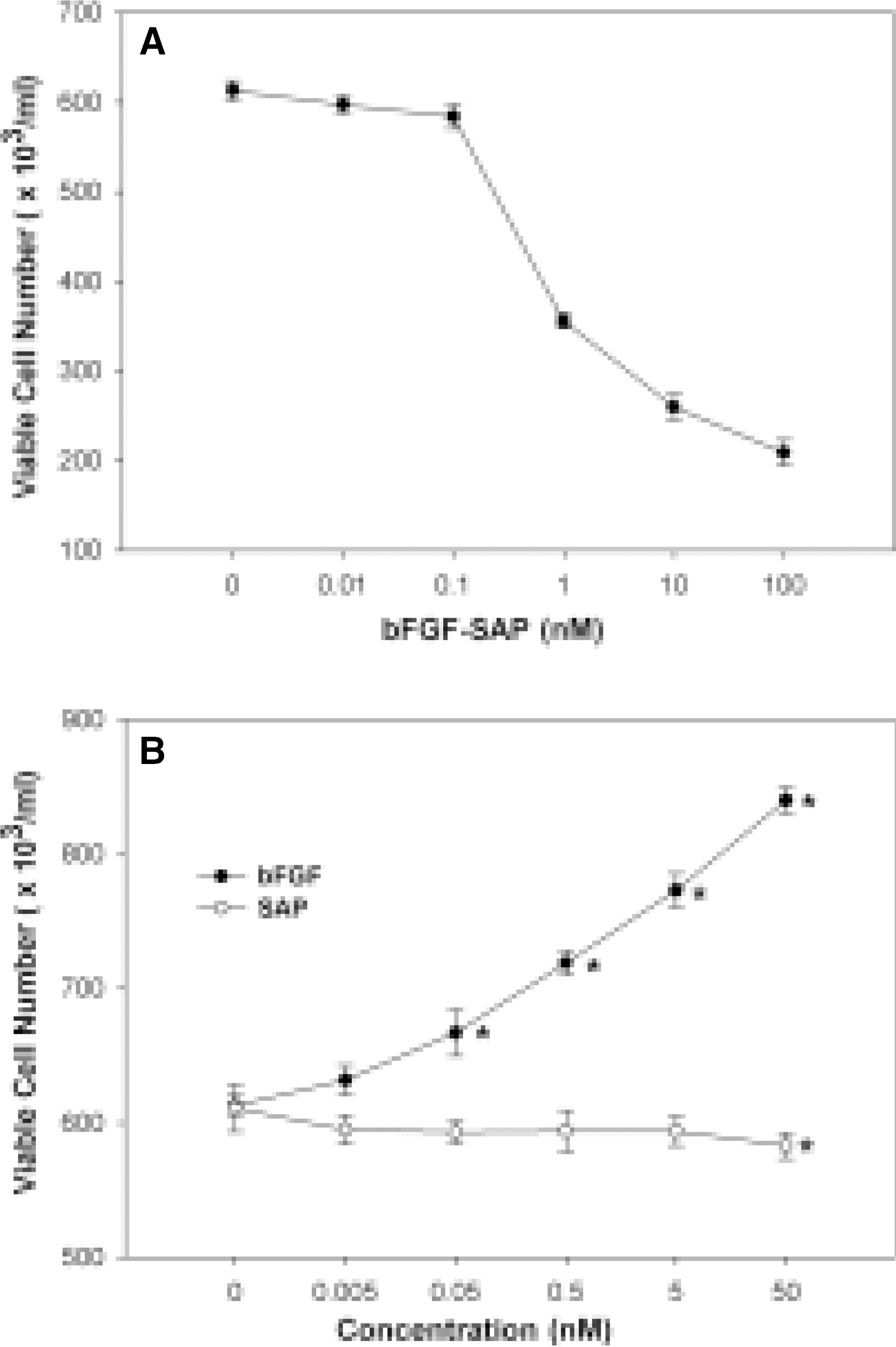
Fig. 3.
Effect of bFGF-SAP, bFGF and saporin on bovine choriocapillary endothelial cells proliferation. A: bFGF-SAP showed dose-dependent inhibition of proliferation. Analysis by nonlinear regression using Sigmaplot V7.0 revealed a typical reverse sigmoid standard curve. ID50 was 0.75 nM. B: bFGF induced proliferation dose-dependently. Saporin showed inhibition of proliferation at a high concentration of 50 nM. Results are given as a mean S.D. ∗P < 0.01 compared with untreated control.
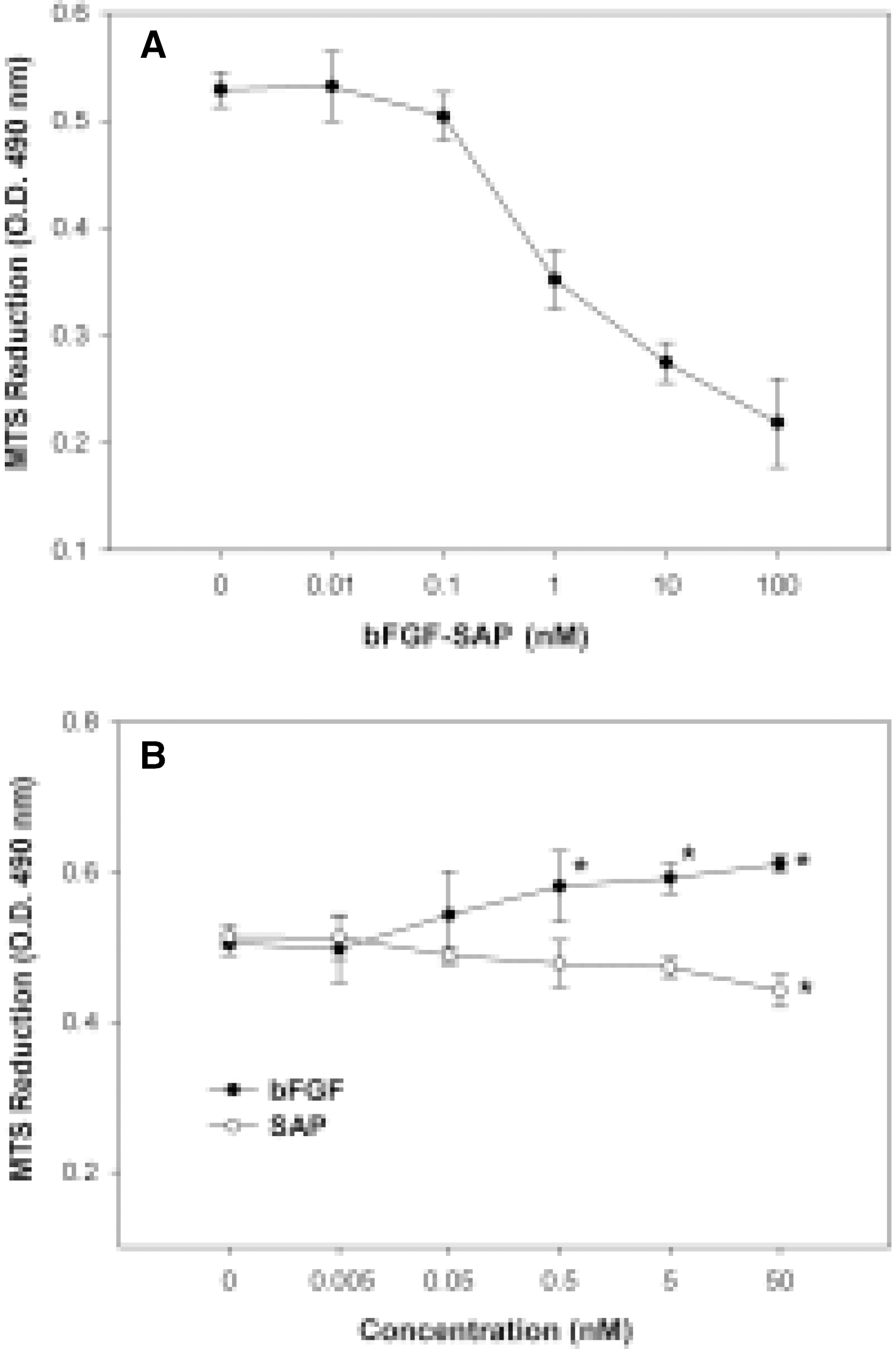
Fig. 4.
Effect of serum on bFGF-SAP-induced inhibition of bovine choriocapillary endothelial cells. In comparison to the 10% FBS group, cell proliferation was decreased in the serum-free group at a given concentration of bFGF-SAP, but still showed a dose-dependent inhibition. The effect of serum was significant. Results are given as a mean S.D. ∗P < 0.01 compared between serum-free and 10% FBS at a given concentration of bFGF-SAP.
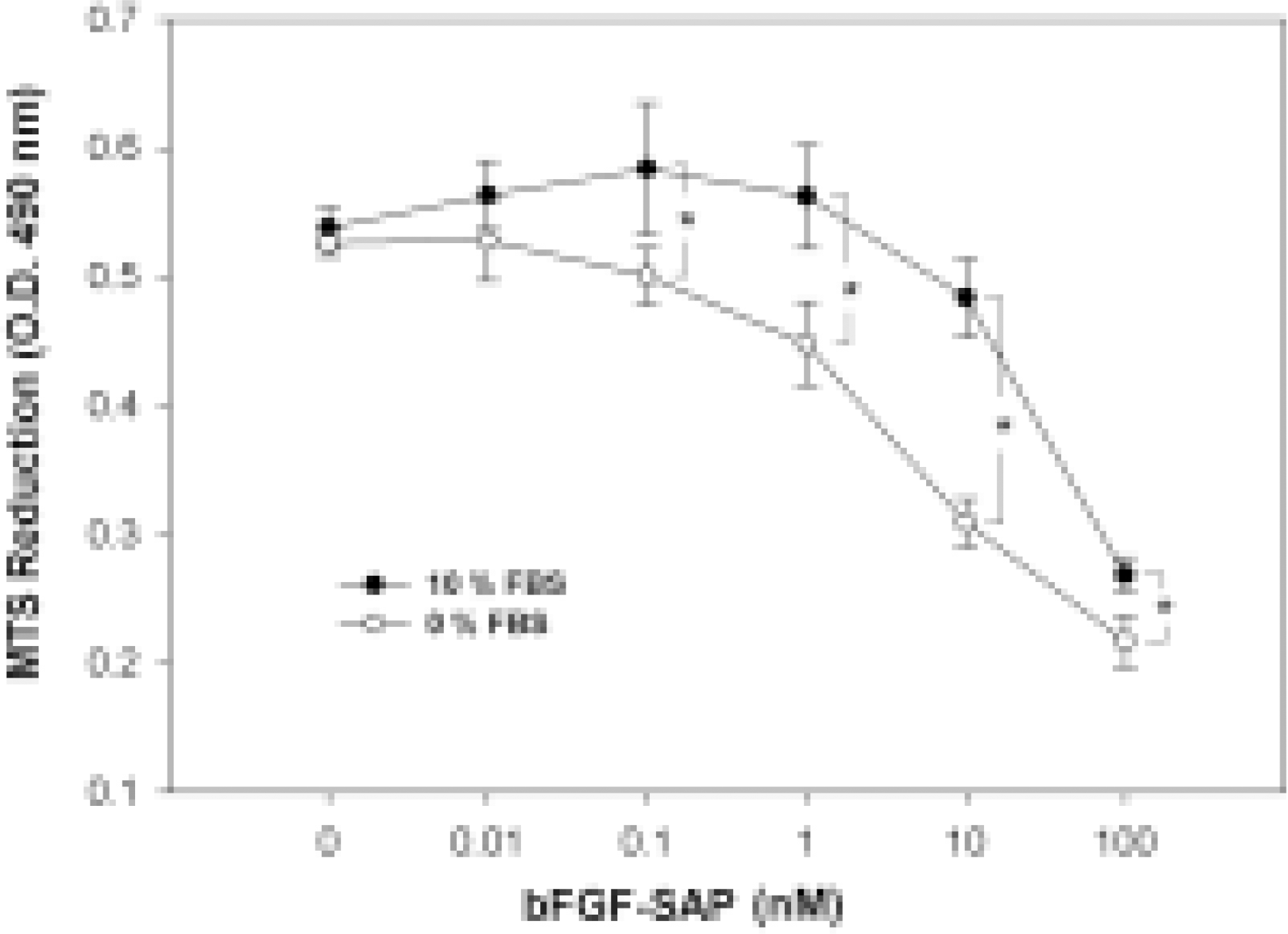
Fig. 5.
bFGF-SAP-induced apoptosis of bovine choriocapillary endothelial cells. A: Control (bFGF-SAP-free). B: bFGF-SAP (10 nM). Cells positive for the TUNEL apoptosis marker were seen as green fluorescein labeled nuclei. (magnification, ×20).
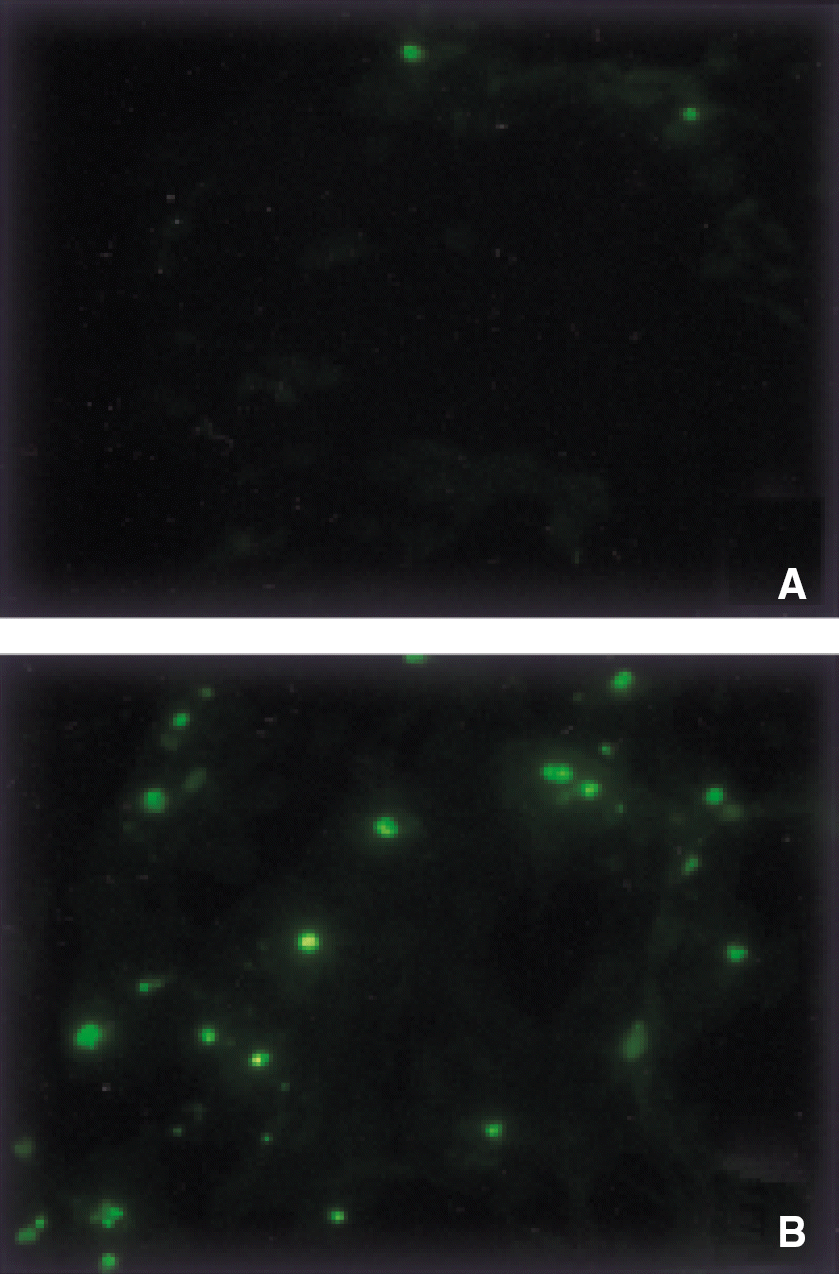
Fig. 6.
Migration assay of bovine choriocapillary endothelial cells. Migration area represents cellular migration indirectly. The control group showed a migration area of 85.48%. bFGF-SAP showed a migration area of 73.66 ± 4.09, 61.68 ± 5.07 and 56.21 ± 4.79% at the concentration of 1, 10 and 100 nM, respectively. Inhibition rate was dose-dependently increased, showing 13.8, 27.7 and 34.3% inhibition at 1, 10 and 100 nM of bFGF-SAP, respectively. Results are given as a mean S.D. ∗P < 0.01 compared with untreated control.
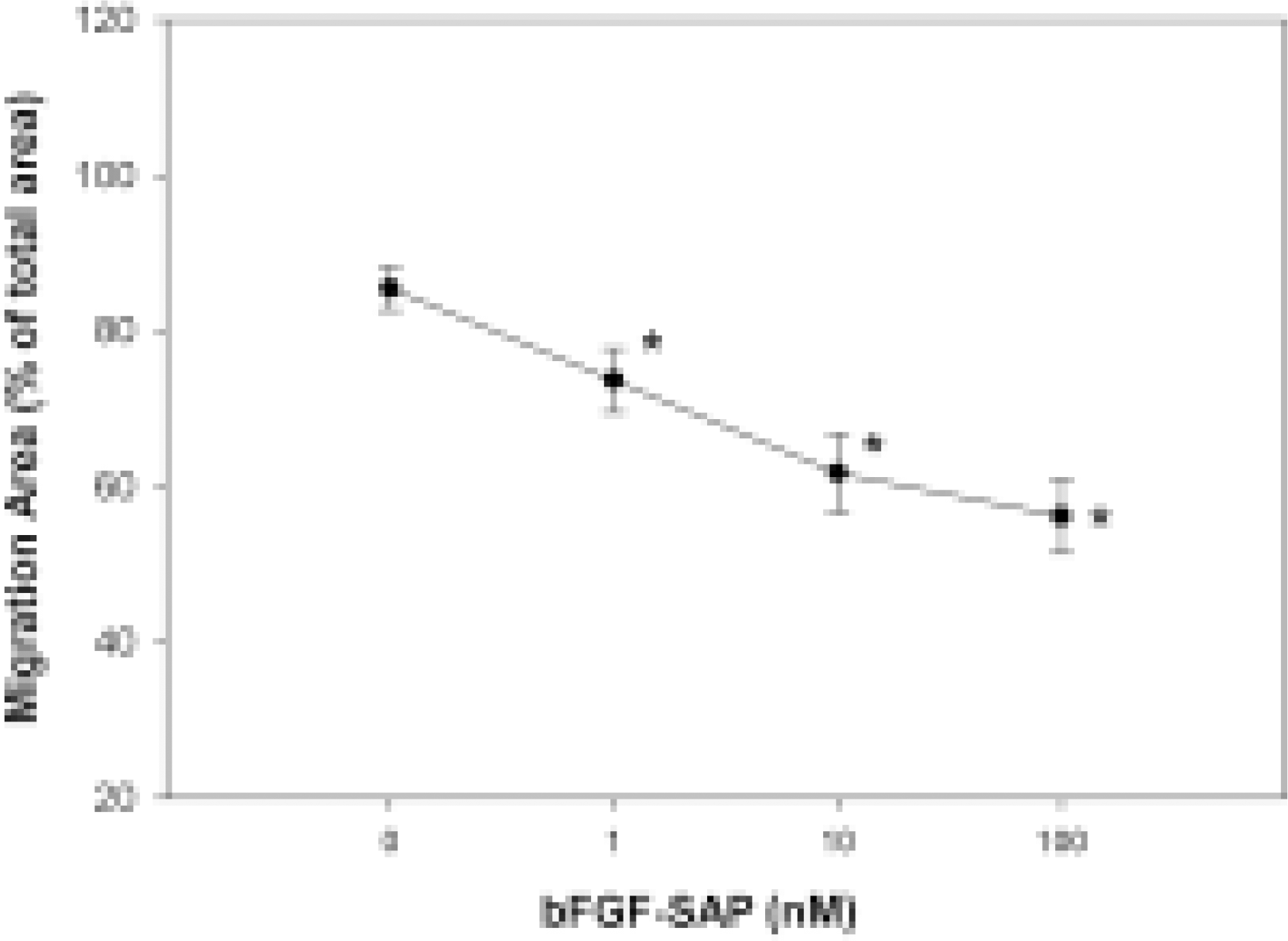
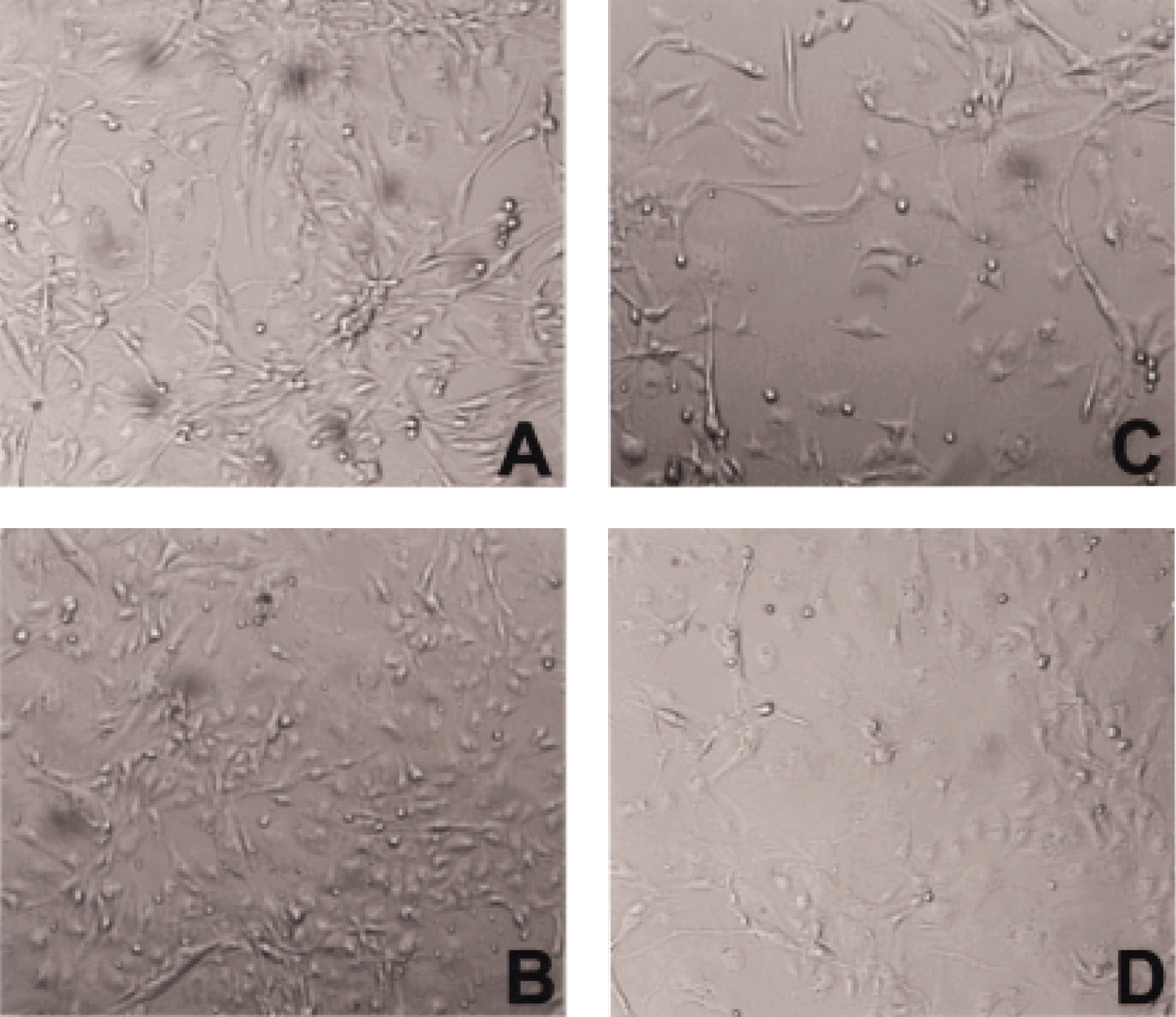
Fig. 7.
Phase contrast photomicrograph of bovine choriocapillary endothelial cell migration. Migration area was dose-dependently decreased by bFGF-SAP. A: Initial control (bFGF-SAP-free) at day 0. B: Control after 72 hours culture. C: bFGF-SAP (1 nM). D: bFGF-SAP (10 nM). E: bFGF-SAP (100 nM). (magnification, ×10).
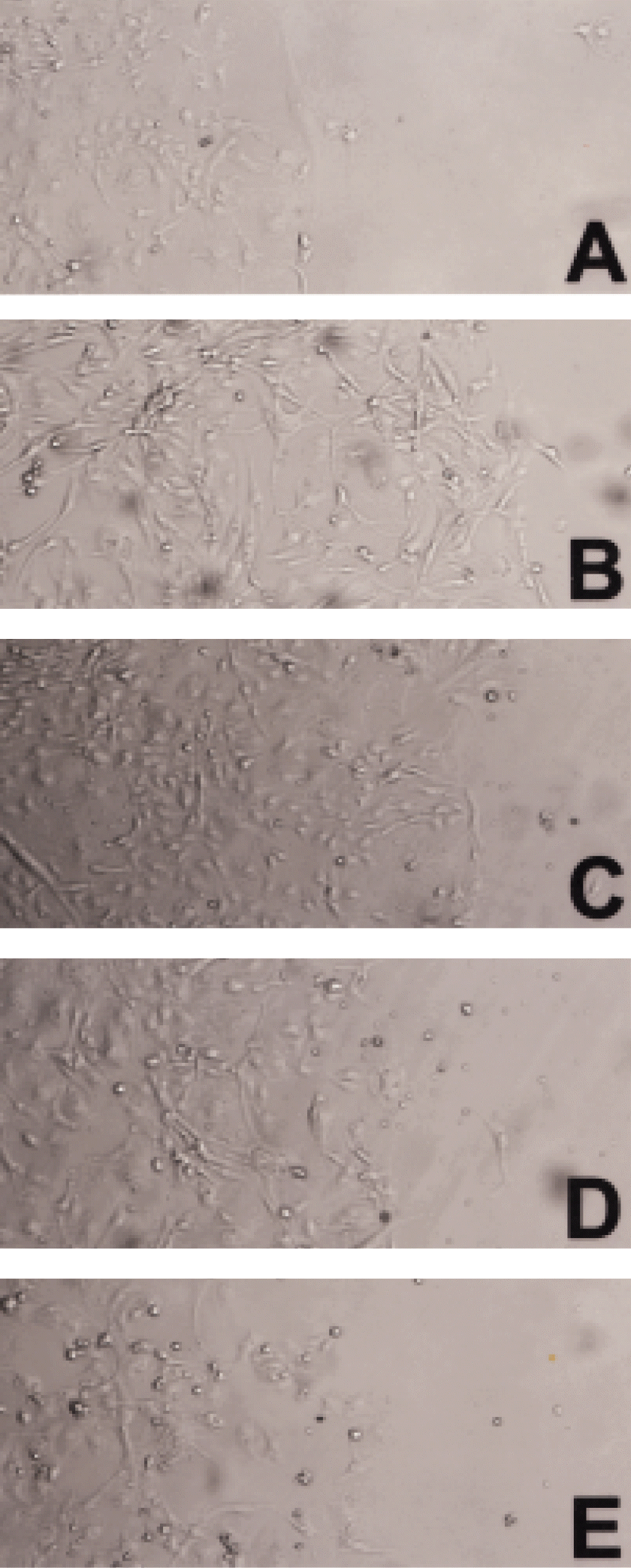
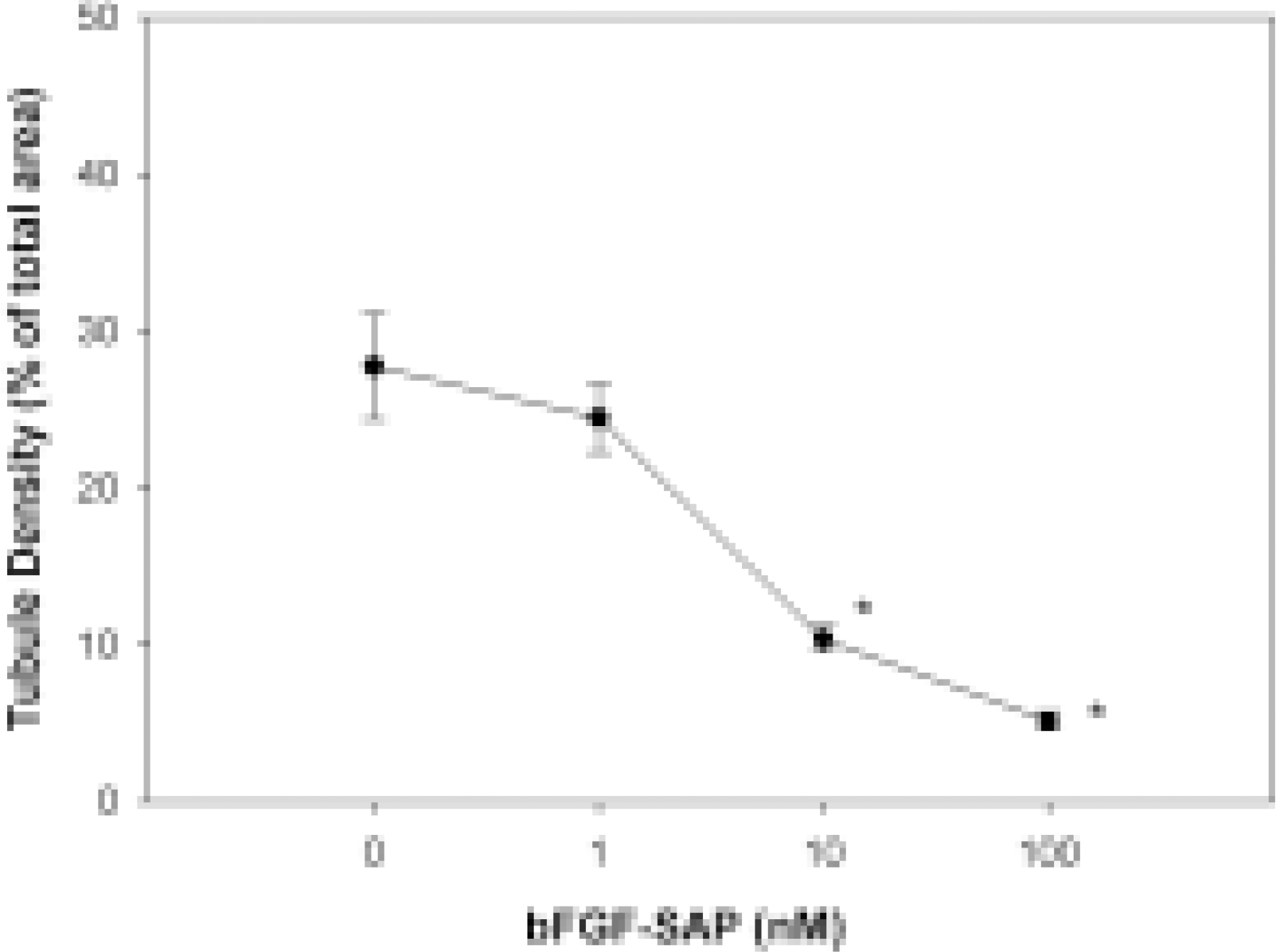
Fig. 8.
Tubule formation assay of bovine choriocapillary endothelial cells. Tubule density represents tubule formation indirectly. The control group showed a tubule area of 27.75 ± 3.37%. bFGF-SAP showed a tubule density of 24.46 ± 2.18, 10.38 ± 0.84 and 5.15 ± 0.56% at the concentration of 1, 10 and 100 nM, respectively. Inhibition rate was dose-dependently increased, showing 11.84, 62.58 and 81.42% inhibition at 1, 10 and 100 nM of bFGF-SAP, respectively. Results are given as a mean S.D. ∗P < 0.01 compared with untreated control.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download