INTRODUCTION
CASE DESCRIPTION
History
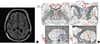
Examination
Fig. 1

Operation
Fig. 2
Postoperative course
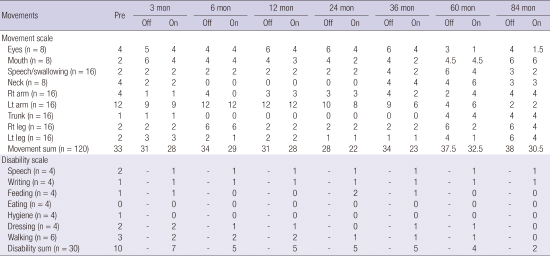
Table 1
The movement and disability scores during the follow periods

Table 2
The stimulation parameters during the follow up periods

Journal List > J Korean Med Sci > v.32(1) > 1023451




AUTHOR CONTRIBUTION Study concept and design of article: Park HR, Jeon BS, Paek SH. Data collection and analysis: Park HR, Lee JM, Park H, Shin CW, Kim HJ. Writing draft: Park HR, Park H, Kim DG, Jeon BS, Paek SH. Revision: Park HR, Lee JM, Park H, Shin CW. Approval of final manuscript and agreement of submission: all authors.
Hye Ran Park 
https://orcid.org/http://orcid.org/0000-0003-0506-4882
Jae meen Lee 
https://orcid.org/http://orcid.org/0000-0002-5708-1644
Hyeyoung Park 
https://orcid.org/http://orcid.org/0000-0003-3504-4612
Chae Won Shin 
https://orcid.org/http://orcid.org/0000-0002-4157-492X
Han-Joon Kim 
https://orcid.org/http://orcid.org/0000-0001-8219-9663
Hee Pyoung Park 
https://orcid.org/http://orcid.org/0000-0002-4772-0780
Dong Gyu Kim 
https://orcid.org/http://orcid.org/0000-0002-5740-6189
Beom Seok Jeon 
https://orcid.org/http://orcid.org/0000-0003-2491-3544
Sun Ha Paek 
https://orcid.org/http://orcid.org/0000-0003-3007-8653