The Republic of Korea experienced the largest outbreak of Middle East respiratory syndrome coronavirus (MERS-CoV) infection outside the Arabian Peninsula in 2015, including 186 laboratory-confirmed cases (
12). The reported data suggest that MERS-CoV is most likely to be transmitted as healthcare-associated infections and poses a serious risk for nosocomial outbreak (
123456). During the nosocomial outbreak of MERS, many patients were identified and quarantined by means of active surveillance. Since MERS-CoV is still a fairly new disease, there is a paucity of data regarding the characteristics of and differences between suspected patients whose tests were subsequently negative and laboratory-confirmed cases (
7). Even though potential predictors of MERS-CoV infection among patients with community-acquired pneumonia (CAP) were identified in the previous study performed in Saudi Arabia (
7), information on possible predictors of infection during the nosocomial outbreak of MERS is still lacking. Therefore, we performed this study to identify possible clinical predictors which can differentiate MERS-CoV-positive patients from MERS-CoV-negative patients with acute febrile illness during the nosocomial outbreak.
We performed a case-control study to identify potential clinical predictors and evaluate differences of initial laboratory data including complete blood counts (CBC) with differential count as predictors for MERS-CoV infection. The cases were defined as hospitalized patients with laboratory-confirmed MERS-CoV infection in the Samsung Medical Center (SMC), a 1,950-bed university-affiliated tertiary care center, between May 2015 and July 2015. The controls were selected from the pool of patients, mostly healthcare workers, who were admitted to the SMC with acute febrile illness (body temperature > 38°C) suspected of viral infection during the same period of the outbreak. The confirmatory diagnostic testing for patients suspected to have MERS-CoV infection was performed as reported previously (
8), and the controls were classified when the MERS-CoV test was negative. Patients were excluded if they did not have epidemiologic links with the nosocomial outbreak in the SMC, or if initial blood tests were not done in the SMC. Patients who were transferred from other hospitals and those who had serious underlying diseases such as solid cancer, hematologic malignancy, life-threatening uncontrolled infections, congestive heart failure or acute myocardial infarction were also excluded in the study population (
Fig. 1). We obtained the following data for each patient from electronic medical records; age, gender, clinical symptoms, underlying diseases, and initial laboratory findings including CBC with differential count, total bilirubin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), and C-reactive protein (CRP) levels. Student’s t-tests or Mann-Whitney U tests were used to compare continuous variables, and χ
2 tests or Fisher’s exact tests were used to compare categorical variables. All
P values were two-tailed, and those < 0.05 were considered to be statistically significant. IBM SPSS Statistics version 20.0 for Windows (IBM, Armonk, NY, USA) was used for all statistical analyses.
Of 45 patients with MERS-CoV infection who were admitted to the SMC, a total of 30 cases were included in the study, and compared with 43 controls whose MERS-CoV test was negative. Demographic data and baseline characteristics of the study population are presented and compared between the case and control groups in
Table 1. The mean ages of the case and control patients were 43.8 and 32.5 years, respectively. The proportion of male patients in the case group was higher than in the control group (60.0% vs. 28.3%;
P = 0.006). There were no significant differences in the underlying diseases except cardiovascular diseases (13.3% vs. 0%;
P = 0.025) and hypertension (16.7% vs. 0%;
P = 0.009). At the time of hospital admission, newly developed cough, sputum, and dyspnea were more frequently seen among the cases than the controls (36.7% vs. 11.6%;
P = 0.011), whereas diarrhea were more frequently seen among the control group (2.0% vs. 55.8%;
P < 0.001).
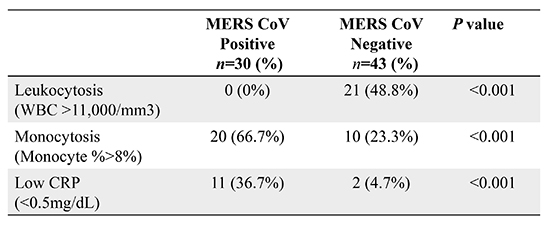
Table 1 also shows the comparison of initial laboratory data for the cases and controls. On the admission, patients with MERS-CoV infection were more likely to have monocytosis (> 8% of total white blood cell [WBC]) with normal WBC count (66.7% vs. 23.3%; odds ratio [OR], 6.600; 95% confidence interval [CI], 2.338–18.629;
P < 0.001) and elevated AST over 40 U/L (26.7% vs. 7.0%; OR, 4.848; 95% CI, 1.166–20.164;
P = 0.021). In contrast, leukocytosis (0% vs. 48.8%; OR, 0.512; 95% CI, 0.382–0.685;
P < 0.001) with relative lymphopenia (30% vs. 100%; OR, 0.300; 95% CI, 0.174–0.518;
P < 0.001) were more frequently seen in the control group. In addition, CRP level lower than 0.5 mg/dL was more seen in the case group than in the control group (36.7% vs. 4.7%; OR, 11.868; 95% CI, 2.392–58.892;
P < 0.001) and median CRP level was significantly lower in the case group than in the control group (0.76 vs. 1.82 mg/dL;
P < 0.001). Lymphocytosis was not seen in both groups and there were no significant differences in hemoglobin level and platelet counts. All patients in the control groups recovered spontaneously without antimicrobial treatment.
In the current study, we found that simple laboratory data such as CBC with differential count could be a useful marker for the prediction of MERS-CoV infection during the nosocomial outbreak. During the nosocomial outbreak of MERS-CoV infection, an unexpected exposure of patients and health care workers can occur, and strict infection control measures are important for the prevention of MERS-CoV spread. Identification of clinical predictors for MERS-CoV infection would be useful in triaging patients into risk categories to determine the likelihood of MERS-CoV infection (
7).
The clinical presentations associated with MERS-CoV infection ranged from mild to fulminant, similar to severe acute respiratory syndrome (SARS) (
91011). The initial symptoms in the most MERS cases were nonspecific fever, usually accompanied by cough, sore throat, myalgia, headache, and diarrhea. However, these symptoms are also common in other viral infections (
13578). Common laboratory findings of MERS were also similar to those of SARS and other respiratory viral infections, and lymphopenia was observed generally among MERS patients (
34812). A recent study noted that MERS-confirmed patients were more likely to have normal WBC count than patients with CAP other than MERS-CoV infection (
7). However, these findings may not be applicable to discriminate MERS from acute febrile illness other than MERS because only patients with CAP were included in the previous study (
7). Furthermore, there were few reports regarding specific laboratory features as predictors for MERS-CoV infection in acute febrile patients without pneumonia during the nosocomial outbreak. Several studies reported that initial laboratory results including normal WBC with lymphopenia and thrombocytopenia were common among SARS patients (
10111314). These laboratory features allowed early classification of febrile patients into likely and unlikely SARS group to utilize limited isolation facilities effectively (
910111314).
Simple clinical markers of host inflammatory responses may be WBC count with differential and CRP. These variables, even if often non-specific, give important information to the clinician and help to decide diagnosis and treatment strategy (
12). The current study suggests that acute febrile patients with recent contact history of MERS, travel history to Middle East, or working history at a hospital affected by MERS could be screened for the possibility of MERS by means of initial laboratory features (e.g. monocytosis with normal WBC count). Initial CRP level was significantly lower in the case patients with MERS-CoV than in the control group without MERS, at least in the initial presentation, and it can be also helpful to predict the possibility of MERS-CoV infection.
Our study has several limitations. First, clinical and laboratory data were retrospectively collected through electronic medical records, and thus unknown risk factors and bias might have been unequally distributed between the two groups, although cases were enrolled prospectively by means of active surveillance of outbreaks. Second, our findings are not generalizable to immunocompromised patient and those with pneumonia, because most patients in this study were immunocompetent hosts without pneumonia. Finally, these laboratory findings suggested in our study are not the definite diagnostic method for MERS-CoV, and it may only be the adjunctive tool for quarantine strategy during the MERS hospital outbreak. Despite these shortcomings, we believe that our study provides clinically useful findings for infection control strategy, especially in the MERS nosocomial outbreak setting.
In conclusion, our study suggests that initial laboratory findings of patients with acute febrile illness could be potential predictors for MERS-CoV infection during the nosocomial outbreak. Monocytosis with normal WBC count and low CRP level may be useful markers for the prediction of MERS and triage at the initial presentation of acute febrile patients.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download