1. Bly R, Jahnen A, Järvinen H, Olerud H, Vassileva J, Vogiatzi S. Collective effective dose in Europe from X-ray and nuclear medicine procedures. Radiat Prot Dosimetry. 2015; 165:129–132.
2. Lee MC, Oh SW, Chung JK, Lee DS. The current status and future perspectives of nuclear medicine in Korea. Nucl Med Mol Imaging. 2010; 44:95–101.
4. ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP Publication 106. Approved by the Commission in October 2007. Ann ICRP. 2008; 38:1–197.
5. Stamm G, Nagel HD. CT-expo--a novel program for dose evaluation in CT. Rofo. 2002; 174:1570–1576.
6. ICRP. Recommendations of the International Commision on Radiological Protection. ICRP Publication 60. Ann ICRP. 1991; 21:1–201.
7. Linet MS, Slovis TL, Miller DL, Kleinerman R, Lee C, Rajaraman P, Berrington de Gonzalez A. Cancer risks associated with external radiation from diagnostic imaging procedures. CA Cancer J Clin. 2012; 62:75–100.
8. Jahnen A, Järvinen H, Olerud H, Vassilieva J, Vogiatzi S, Shannoun F, Bly R. Analysis of factors correlating with medical radiological examination frequencies. Radiat Prot Dosimetry. 2015; 165:133–136.
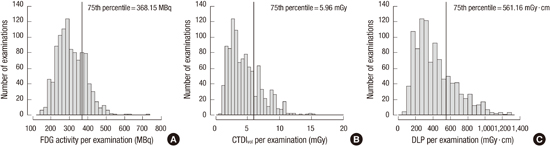
10. Etard C, Celier D, Roch P, Aubert B. National survey of patient doses from whole-body FDG PET-CT examinations in France in 2011. Radiat Prot Dosimetry. 2012; 152:334–338.
11. Avramova-Cholakova S, Ivanova S, Petrova E, Garcheva M, Vassileva J. Patient doses from PET-CT procedures. Radiat Prot Dosimetry. 2015; 165:430–433.
12. Rausch I, Bergmann H, Geist B, Schaffarich M, Hirtl A, Hacker M, Beyer T. Variation of system performance, quality control standards and adherence to international FDG-PET/CT imaging guidelines. A national survey of PET/CT operations in Austria. Nucl Med (Stuttg). 2014; 53:242–248.
13. Boellaard R, O’Doherty MJ, Weber WA, Mottaghy FM, Lonsdale MN, Stroobants SG, Oyen WJ, Kotzerke J, Hoekstra OS, Pruim J, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2010; 37:181–200.
14. Boellaard R, Delgado-Bolton R, Oyen WJ, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015; 42:328–354.
15. Karp JS, Surti S, Daube-Witherspoon ME, Muehllehner G. Benefit of time-of-flight in PET: experimental and clinical results. J Nucl Med. 2008; 49:462–470.
16. Akamatsu G, Ishikawa K, Mitsumoto K, Taniguchi T, Ohya N, Baba S, Abe K, Sasaki M. Improvement in PET/CT image quality with a combination of point-spread function and time-of-flight in relation to reconstruction parameters. J Nucl Med. 2012; 53:1716–1722.
17. Boellaard R, Oyen WJ, Hoekstra CJ, Hoekstra OS, Visser EP, Willemsen AT, Arends B, Verzijlbergen FJ, Zijlstra J, Paans AM, et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur J Nucl Med Mol Imaging. 2008; 35:2320–2333.
18. Graham MM, Wahl RL, Hoffman JM, Yap JT, Sunderland JJ, Boellaard R, Perlman ES, Kinahan PE, Christian PE, Hoekstra OS, et al. Summary of the UPICT protocol for 18F-FDG PET/CT imaging in oncology clinical trials. J Nucl Med. 2015; 56:955–961.
19. ICRP. International Commission on Radiological Protection, Committee I. The evaluation of risks from radiation. Health Phys. 1966; 12:239–302.
20. European Commission. Group of Advisers on the Ethical Implications of Biotechnology. Report to the European Commission: ethical aspects of cloning techniques. Politics Life Sci. 1997; 16:309–312.
21. Botros GM, Smart RC, Towson JE. Diagnostic reference activities for nuclear medicine procedures in Australia and New Zealand derived from the 2008 survey. ANZ Nucl Med. 2009; 40:2–11.
22. Korpela H, Bly R, Vassileva J, Ingilizova K, Stoyanova T, Kostadinova I, Slavchev A. Recently revised diagnostic reference levels in nuclear medicine in Bulgaria and in Finland. Radiat Prot Dosimetry. 2010; 139:317–320.









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download