INTRODUCTION
CURRENT STATUS OF NUCLEAR MEDICINE PRACTICE IN KOREA
Gamma camera imaging
Table 1
Diagnostic nuclear medicine examinations using gamma camera in 2013 done in Korea

Table 2
Diagnostic nuclear medicine examinations using SPECT/CT in 2013 done in Korea
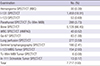
PET imaging
Table 3
Diagnostic nuclear medicine examinations using PET(/CT) in 2013 done in Korea
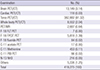
Therapies with radiopharmaceuticals
Table 4
Radiopharmaceutical therapies performed in 2013 in Korea

IMPLEMENTATION OF RADIOLOGICAL JUSTIFICATION FOR NUCLEAR MEDICINE PRACTICE
Recommendations of the ICRP for justification for medical exposure to radiation
Table 5
The ICRP recommendations on radiological justification and optimization in medicine

Implementation of radiological justification for nuclear medicine practice by international professional bodies
Table 6
Referral guidelines for implementation of radiological justification in nuclear medicine

| Professional bodies | Guidelines |
|---|---|
| International professional bodies | SNMMI; evidence-based referral guidelines |
| Oncology Practice Guidelines Summary from multiple organizations for major cancer Types (2,3,4,5,6) | |
| Cardiac Practice Guidelines Summary (7) | |
| ACR; evidence-based rating referral guidelines to assist in making the most appropriate imaging for a specific clinical condition | |
| ACR's Appropriateness Criteria® (8) | |
| ATA; evidence-based rating referral guideline through collaboration with related academic societies | |
| Revised American Thyroid Association Management Guidelines for Patients with Thyroid Nodules and Differentiated Thyroid Cancer (2009) (9) | |
| Korean professional bodies | KSNM; evidence-based rating referral guidelines |
| Clinical application of 18F-FDG PET in various cancer (10) | |
| Clinical application of 18F-FDG PET in Neurology (27) | |
| KTA; evidence-based rating referral guidelines through collaboration with related academic societies | |
| Revised Management Guidelines for Patients with Thyroid Nodules and Thyroid Cancer (11) |
Implementation of justification for nuclear medicine practice in Korea
IMPLEMENTATION OF RADIOLOGICAL OPTIMIZATION FOR NUCLEAR MEDICINE PRACTICE
Recommendations of the ICRP for optimization of protection to medical exposure to radiation
Implementation of radiological optimization for nuclear medicine practice by international professional bodies
Table 7
Guidelines for radiopharmaceuticals dose optimization in nuclear medicine

| Professional bodies | Guidelines |
|---|---|
| International professional bodies | ACR-AAPM; guideline presents diagnostic reference levels |
| ACR-AAPM Practice Parameter for Reference Levels and Achievable Administered Activity for Nuclear Medicine and Molecular Imaging (14) | |
| cf) Reference Levels is based on NCRP partial national survey data. | |
| SNMMI; Procedure Standards containing dose recommendations, equipment QC, personnel qualification (15) | |
| SNMMI-EANM; harmonized pediatric dose guideline | |
| Pediatric radiopharmaceutical administration: harmonization of the 2007 EANM pediatric dosage card (version 1.5.2008) and the 2010 North American consensus guidelines (17) | |
| IAC Nuclear/PET; standard for PET accreditation | |
| The IAC Standards and Guidelines for Nuclear/PET Accreditation (18) | |
| Korean professional bodies | Regarding DRLs; survey is under progress |
| KSNM starts surveying Individual hospital protocols for calculation of administered doses through the KSNM website. | |
| KSNM is developing a survey method for CT Dose Index (CTDI) and Dose Length Products (DLP) | |
| KSNM; procedure guidelines including dose recommendations | |
| Nuclear Medicine Procedure Manual (20) | |
| 18F-FDG PET Procedure Standard (21) | |
| KSNM; technical standard for radiopharmaceuticals related personnel and facilities | |
| KSNM technical standard for procedures using radiopharmaceuticals (28) | |
| Regarding accreditation; relevant foundation is under progress | |
| KARA and other agencies have been performing certification services | |
| A foundation tentatively named the "Institute for Quality Management of Nuclear Medicine," is being established |
Implementation of diagnostic reference levels for nuclear medicine practice in Korea
IMPLEMENTATION OF PROTECTION FOR CAREGIVERS AND COMFORTERS OF PATIENTS TREATED WITH RADIONUCLIDES
The recommendations of ICRP for protection of caregivers and comforters of patients treated with radionuclides
Implementation of protection for caregivers and comforters of patients treated with radionuclides by international professional bodies
Table 8
Guidelines for implementation of protection for caregivers and comforters of patients treated with radionuclide

| Organization | Guidelines |
|---|---|
| ICRP | The 2007 Recommendation; dose constraint for caregivers and comforters |
| 5 mSv per episode | |
| 1 mSv for young children, infants, and visitors | |
| International professional bodies | IAEA; Patient instruction card containing restriction periods determined by each hospital |
| https://rpop.iaea.org/RPOP/RPoP/Content/Documents/Whitepapers/patient-information.pdf (24) | |
| ATA; guideline presents restricted periods according to the administered activities | |
| Korean professional bodies | Radiation Safety Guideline for I-131 Thyroid cancer therapy (25) |
| MRSRC; radiation safety instruction for I-131 Thyroid cancer therapy presents restricted periods according to the administered activities | |
| Radiation Safety Instruction for I-131 Thyroid cancer therapy (26) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download