1. Malone J, Guleria R, Craven C, Horton P, Järvinen H, Mayo J, O’Reilly G, Picano E, Remedios D, Le Heron J, et al. Justification of diagnostic medical exposures: some practical issues. Report of an International Atomic Energy Agency Consultation. Br J Radiol. 2012; 85:523–538.
2. International Atomic Energy Agency (IAEA). Report of a consultation on justification of patient exposures in medical imaging. Radiat Prot Dosimetry. 2009; 135:137–144.
3. Malone J, O’Connor U, Faulkner K. SENTINEL Project special initiative: ethical and justification issues in medical radiation protection. Radiat Prot Dosimetry. 2009; 135:69–70.
4. National Research Council (US) Committee on Health Effects of Exposure to Low Levels of Ionizing Radiations. Health effects of exposure to low levels of ionizing radiations: time for reassessment? Washington, D.C.: National Academy Press;1998.
5. International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP. 2007; 37:1–332.
6. Medina LS, Blackmore CC. Evidence-based radiology: review and dissemination. Radiology. 2007; 244:331–336.
7. Sardanelli F, Hunink MG, Gilbert FJ, Di Leo G, Krestin GP. Evidence-based radiology: why and how? Eur Radiol. 2010; 20:1–15.
8. Royal College of Radiologists Working Party. Influence of Royal College of Radiologists’ guidelines on referral from general practice. BMJ. 1993; 306:110–111.
9. Eccles M, Steen N, Grimshaw J, Thomas L, McNamee P, Soutter J, Wilsdon J, Matowe L, Needham G, Gilbert F, et al. Effect of audit and feedback, and reminder messages on primary-care radiology referrals: a randomised trial. Lancet. 2001; 357:1406–1409.
10. Oakeshott P, Kerry SM, Williams JE. Randomized controlled trial of the effect of the Royal College of Radiologists’ guidelines on general practitioners’ referrals for radiographic examination. Br J Gen Pract. 1994; 44:197–200.
11. Hsu J, Brożek JL, Terracciano L, Kreis J, Compalati E, Stein AT, Fiocchi A, Schünemann HJ. Application of GRADE: making evidence-based recommendations about diagnostic tests in clinical practice guidelines. Implement Sci. 2011; 6:62.
12. Gopalakrishna G, Mustafa RA, Davenport C, Scholten RJ, Hyde C, Brozek J, Schünemann HJ, Bossuyt PM, Leeflang MM, Langendam MW. Applying Grading of Recommendations Assessment, Development and Evaluation (GRADE) to diagnostic tests was challenging but doable. J Clin Epidemiol. 2014; 67:760–768.
18. Moriarity AK, Klochko C, O’Brien M, Halabi S. The effect of clinical decision support for advanced inpatient imaging. J Am Coll Radiol. 2015; 12:358–363.
20. Remedios D, Hierath M, Ashford N, Bezzi M, Cavanagh P, Chateil JF, Grenier P, Simeonov G, Vilgrain V. Imaging referral guidelines in Europe: now and in the future-EC Referral Guidelines Workshop Proceedings. Insights Imaging. 2014; 5:9–13.
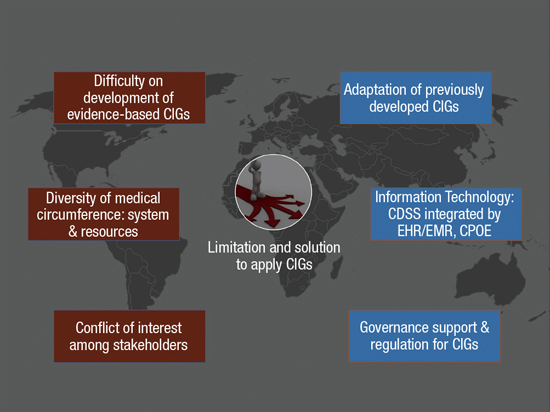
21. Malone J, del Rosario-Perez M, Van Bladel L, Jung SE, Holmberg O, Bettmann MA. Clinical imaging guidelines part 2: Risks, benefits, barriers, and solutions. J Am Coll Radiol. 2015; 12:158–165.
23. Kassing P, Duszak R. Repeat medical imaging: a classification system for meaningful policy analysis and research. Reston, VA: Harvey L. Neiman Health Policy Institute;2013. p. 1–7.






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download