INTRODUCTION
MATERIALS AND METHODS
Subjects
Family 1
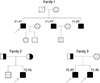 | Fig. 1Pedigrees of affected individuals with SLCO2A1 mutations. In the pedigree, arrows indicate the proband. Filled black, patients with PDP; Half-black, healthy members with a heterozygous mutation; Gray, healthy members not doing genotyping test. |
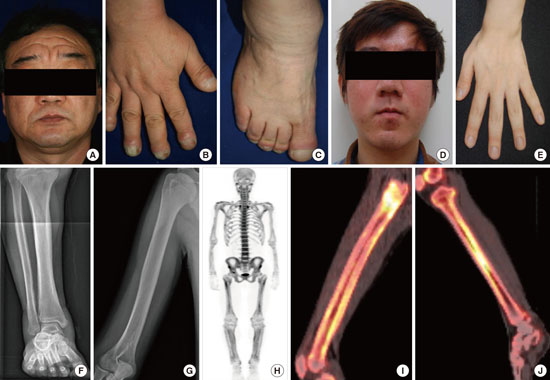
 | Fig. 2Clinical features of affected individuals with SLCO2A1 mutations.
(A-C) Clinical pictures of family 1 proband. (A) Thickening and furrowing of the facial skin. (B-C) Digital clubbing and swelling of the ankle joint. (D-H) Clinical pictures of family 2 proband. (D) Thickening and greasiness of facial skin. (E) Digital clubbing. (F-G) Cortical hyperostosis of long bones. (H) Diffusely increased uptake in the whole axial and appendicular long bones shown by whole body bone scan. (I-J) 18F-fluoride PET scan images of femur and tibia show increased cortical/periosteal uptake in the proband of family 3.
|
Family 2
Family 3
Mutation analysis of the associated genes
RESULTS
Clinical features of PDP patients
Table 1
Clinical phenotypes of Korean patients with pachydermoperisostasis

| Phenotypes | Cases | |||||
|---|---|---|---|---|---|---|
| F1-P1 | F1-P2 | F1-P3 | F2-P4 |
F3-P5 Kim et al. (10) |
F3-P6 Kim et al. (10) |
|
| Current age, yr | 56 | 54 | 52 | 19 | 23 | 19 |
| Onset age, yr | 19 | 17 | 20 | 17 | puberty | 13 |
| Gender | M | M | M | M | M | M |
| SLCO2A1 mutations | c.302T>G | c.302T>G | c.302T>G | c.940+1G>A | c.940+1G>A | c.940+1G>A |
| c.1807C>T | c.940+1G>A | c.940+1G>A | ||||
| Triad | ||||||
| Digital clubbing | + | + | + | + | + | + |
| Periostosis | + | + | + | + | + | + |
| Pachydermia | + | + | + | + | + | + |
| Skin | ||||||
| Palmar and plantar hyperhidrosis | + | - | - | - | - | - |
| Acne | + | + | + | + | + | + |
| Seborrhoea and eczema | + | + | + | + | + | + |
| Skeletal | ||||||
| History of bone fractures | - | - | - | - | - | - |
| Swelling of large joints | + | + | + | + | + | + |
| Painful joints on exercise | + | + | + | + | + | + |
| Hydrarthrosis | + | + | + | + | + | + |
| Others | ||||||
| Anemia | - | - | - | - | - | - |
| Hypoalbuminemia | - | - | - | - | - | - |
| Patent ductus arteriosus | - | - | - | + | - | + |
Identification of SLCO2A1 as a gene responsible for PDP
 | Fig. 3Localization and sequence chromatogram of identified SLCO2A1 mutations. Upper: The positions of the mutations in the exons of SLCO2A1 in this study. Lower: SLCO2A1 mutations in PDP families. family 1, (A) c.302T>G; family 2, (B) c.940+1G>A and (C) c.1807C>T; family 3, (C) c.940+1G>A. |
DISCUSSION
Table 2
Summary of the genetic SLCO2A1 mutations in patients with pachydermoperisostasis in the literature
| References | Mutation | Origin | Sex | Age | Clubbing | Periostosis | Pachydermia | Hyperhidrosis | Seborrhea | Arthralgia | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhang et al. (19) | c.940+1G>A | c.1602C>A | Chinese | M | 22 | + | + | + | + | NA | + |
| Zhang et al. (3) | c.855delA | c.855delA | Chinese | M | 36 | + | + | + | - | + | + |
| c.855delA | c.855delA | Chinese | F | 47 | - | - | - | - | - | - | |
| c.855delA | c.855delA | Chinese | F | 42 | - | - | - | - | - | - | |
| c.1106G>A | c.1106G>A | Chinese | M | 23 | + | + | + | + | + | + | |
| c.1393G>A | c.1393G>A | Chinese | M | 26 | + | + | + | - | + | + | |
| c.493G>T | c.1136G>A | Chinese | M | 18 | + | + | + | - | + | + | |
| c.664G>A | c.1634delA | Chinese | M | 24 | + | + | + | - | + | + | |
| c.861+2T>C | Chinese | M | 42 | + | + | + | - | + | + | ||
| c.1065dupA | Chinese | M | 17 | + | + | + | + | + | + | ||
| Zhang et al. (31) | c.235-1G>T | c.656C>T | Chinese | M | 27 | + | + | + | + | NA | + |
| Zhang et al. (6) | c.97-1G>A | c.97-1G>A | Chinese | M | 24 | + | + | + | NA | NA | NA |
| c.764G>A | c.1634delA | Chinese | M | 27 | + | + | + | NA | NA | NA | |
| c.664G>A | c.940+1G>A | Chinese | M | 21 | + | + | + | NA | NA | NA | |
| Cheng et al. (32) | c.547G>A | c.1807C>T | Chinese | M | 25 | + | + | + | + | + | + |
| c.940+1G>A | c.1602C>A | Chinese | M | 37 | + | + | + | NA | + | + | |
| Niizeki et al. (12) | c.940+1G>A | c.1279_1290del12 | Japanese | M | 19 | + | + | + | + | + | - |
| c.754C>T | c.1807C>T | Japanese | M | 21 | + | + | + | + | - | + | |
| c.421G>T | c.940+1G>A | Japanese | M | 20 | + | + | + | + | + | - | |
| c.940+1G>A | c.1807C>T | Japanese | M | 20 | + | + | + | - | + | + | |
| Sasaki et al. (5) | c.940+1G>A | c.1279_1290del12 | Japanese | M | 24 | + | + | + | + | + | + |
| c.310G>A | c.1040C>T | Japanese | M | 25 | + | + | + | + | + | + | |
| c.940+1G>A | c.940+1G>A | Japanese | M | 45 | + | + | + | - | + | - | |
| c.940+1G>A | c.1668G>C | Japanese | M | 53 | + | + | + | - | - | + | |
| Niizeki et al. (9) | c.1279G>A | c.1807C>T | Japanese | F | 67 | + | + | - | - | - | + |
| Minakawa et al. (33) | c.940+1G>A | c.1279_1290del12 | Japanese | M | 15 | + | + | + | + | + | + |
| Busch et al. (14) | c.940+1G>A | c.1668G>C | Japanese | M | 53 | + | + | NA | NA | NA | + |
| c.940+1G>A | c.940+1G>A | Japanese | M | 21 | + | NA | + | NA | NA | NA | |
| c.940+1G>A | c.940+1G>A | Japanese | M | 19 | + | NA | + | NA | NA | NA | |
| c.1292delC | c.1292delC | Indian | M | 27 | + | NA | + | NA | + | + | |
| c.763G>A | c.763G>A | Indian | M | 26 | + | + | NA | + | NA | + | |
| c.763G>A | c.763G>A | Indian | M | 28 | + | + | NA | + | NA | + | |
| Seifert et al. (8) | c.830_831insT | c.830_831insT | Turkish | M | 21 | + | + | + | + | + | + |
| c.830_831insT | c.830_831insT | Turkish | M | 19 | + | + | + | + | + | + | |
| c.830_831insT | c.830_831insT | Turkish | M | 7 | - | - | - | - | - | - | |
| c.830_831insT | Turkish | M | 40 | + | - | - | - | - | - | ||
| c.1670T>C | c.1670T>C | Iraq | M | 38 | + | + | + | + | + | + | |
| c.754C>T | Dutch | M | 28 | + | - | - | - | - | - | ||
| Diggle et al. (7) | c.1259G>T | c.1259G>T | Hispanic (Colombia) | M | 45 | + | + | + | NA | NA | NA |
| c.941-1G>A | c.1517C>A | Chinese | M | NA | + | + | + | NA | NA | NA | |
| c.542G>C | c.542G>C | Turkish | M | NA | + | + | + | NA | NA | NA | |
| c.1333C>T | Dutch | M | NA | + | + | + | NA | NA | NA | ||
| c.290G>A | c.940+2T>A | French | M | NA | NA | NA | NA | NA | NA | NA | |
| c.664G>A | c.664G>A | North African | M | NA | NA | NA | NA | NA | NA | NA | |
| c.253A>T | c.253A>T | North African | M | NA | NA | NA | NA | NA | NA | NA | |
| c.1105+4A>G | c.1105+4A>G | Dutch | M | NA | NA | NA | NA | NA | NA | NA | |
| c.838C>T | c.1693T>G | Kabardin (Caucasus) | M | NA | NA | NA | NA | NA | NA | NA | |
| c.310G>T | c.310G>T | Italian | M | NA | NA | NA | NA | NA | NA | NA | |
| c.724+1G>T | c.724+1G>T | Algerian | M | NA | NA | NA | NA | NA | NA | NA | |
| c.542G>A | c.542G>A | Turkish | M | NA | NA | NA | NA | NA | NA | NA | |
| c.611C>T | c.611C>T | Italian | M | NA | NA | NA | NA | NA | NA | NA | |
| Ayoub et al. (34) | c.1016C>T | c.1016C>T | Saudi | M | 23 | + | + | + | + | + | + |
| Madruga Dias et al. (35) | c.940+1G>A | c.940+1G>A | African | M | 26 | + | + | + | NA | NA | + |
| Saadeh et al. (36) | c.838C>T | c.838C>T | Lebanese | M | 22 | + | + | + | NA | NA | + |
| c.838C>T | c.838C>T | Lebanese | M | 24 | + | NA | + | NA | NA | + | |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download