1. Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L. Hepatitis B: Epidemiology and prevention in developing countries. World J Hepatol. 2012; 4:74.
2. WHO. WHO position paper on hepatitis A vaccines – June 2012. Wkly Epidemiol Rec. 2012; 87:261–276.
3. Kim JH. Recent epidemiological status and vaccination of hepatitis A in Korea. J Korean Med Assoc. 2008; 51:110–118.
4. Li RC, Li Y, Yi N, Huang L, Wan Z, Zhang Y, Rasuli A. An open, prospective, randomized study comparing the immunogenicity and safety of two inactivated hepatitis A pediatric vaccines in toddlers, children and adolescents in China. Pediatr Infect Dis J. 2013; 32:e77–e81.
5. American Academy of Pediatrics Committee on Infectious Diseases. Hepatitis A vaccine recommendations. Pediatrics. 2007; 120:189–199.
6. Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine. 2010; 28:6653–6657.
7. Lee H, Cho HK, Kim JH, Kim KH. Seroepidemiology of hepatitis A in Korea: changes over the past 30 years. J Korean Med Sci. 2011; 26:791–796.
8. Kim YJ, Lee HS. Increasing incidence of hepatitis A in Korean adults. Intervirology. 2010; 53:10–14.
9. Jeong SH. Current status and vaccine indication for hepatitis A virus infection in Korea. Korean J Gastroenterol. 2008; 51:331–337.
10. Key H. World's first hepatitis A vaccine. Inpharma Wkly. 1992; 823:14–15.
11. Murphy TV, Feinstone SM. Hepatitis A vaccines. In : Plotkin SA, editor. Vaccines. Philadelphia, PA: Saunders Elsevier;2013. p. 183–204.
12. Fiore AE, Wasley A, Bell BP. Advisory Committee on Immunization Practices (ACIP). Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2006; 55(RR-7):1–23.
13. Fangcheng Z, Xuanyi W, Mingding C, Liming J, Jie W, Qi J, Yuanping G, Wen Q, Yajuan X, Jiangsen M. Era of vaccination heralds a decline in incidence of hepatitis A in high-risk groups in China. Hepat Mon. 2012; 12:100–105.
14. Vidor E, Dumas R, Porteret V, Bailleux F, Veitch K. Aventis Pasteur vaccines containing inactivated hepatitis A virus: a compilation of immunogenicity data. Eur J Clin Microbiol Infect Dis. 2004; 23:300–309.
15. Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008; 7:1141–1150.
16. Abarca K, Ibáñez I, de la Fuente P, Cerda L, Bergeret J, Frösner G, Ibarra H. Immunogenicity and tolerability of a paediatric presentation of a virosomal hepatitis A vaccine in Chilean children aged 1-16 years. Vaccine. 2011; 29:8855–8862.
17. Bovier PA, Bock J, Ebengo TF, Frösner G, Glaus J, Herzog C, Loutan L. Predicted 30-year protection after vaccination with an aluminum-free virosomal hepatitis A vaccine. J Med Virol. 2010; 82:1629–1634.
18. Abarca K, Ibánez I, Perret C, Vial P, Zinsou JA. Immunogenicity, safety, and interchangeability of two inactivated hepatitis A vaccines in Chilean children. Int J Infect Dis. 2008; 12:270–277.
19. Soysal A, Gokçe I, Pehlivan T, Bakir M. Interchangeability of a hepatitis A vaccine second dose: Avaxim 80 following a first dose of Vaqta 25 or Havrix 720 in children in Turkey. Eur J Pediatr. 2007; 166:533–539.
20. López EL, Contrini MM, Xifró MC, Cattaneo MA, Zambrano B, Dumas R, Rouyrre N, Weber F. Hepatitis A vaccination of Argentinean infants: comparison of two vaccination schedules. Vaccine. 2007; 25:102–108.
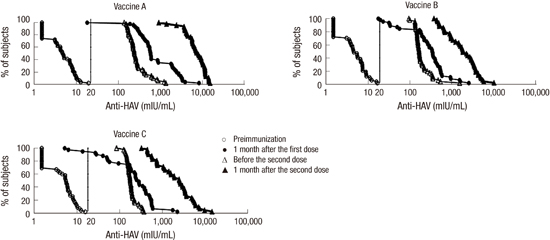
21. Lim J, Song YJ, Park WS, Sohn H, Lee MS, Shin DH, Kim CB, Kim H, Oh GJ, Ki M. The immunogenicity of a single dose of hepatitis A virus vaccines (Havrix® and Epaxal®) in Korean young adults. Yonsei Med J. 2014; 55:126–131.
22. Zuckerman JN, Kirkpatrick CT, Huang M. Immunogenicity and reactogenicity of Avaxim (160 AU) as compared with Havrix (1440 EL.U) as a booster following primary immunization with Havrix (1440 EL.U) against hepatitis A. J Travel Med. 1998; 5:18–22.
23. Orr N, Klement E, Gillis D, Sela T, Kayouf R, Derazne E, Grotto I, Balicer R, Huerta M, Aviram L, et al. Long-term immunity in young adults after a single dose of inactivated Hepatitis A vaccines. Vaccine. 2006; 24:4328–4332.
24. Van Der Wielen M, Vertruyen A, Froesner G, Ibáñez R, Hunt M, Herzog C, Van Damme P. Immunogenicity and safety of a pediatric dose of a virosome-adjuvanted hepatitis A vaccine: a controlled trial in children aged 1-16 years. Pediatr Infect Dis J. 2007; 26:705–710.
25. Dagan R, Amir J, Livni G, Greenberg D, Abu-Abed J, Guy L, Ashkenazi S, Foresner G, Tewald F, Schätzl HM, et al. Concomitant administration of a virosome-adjuvanted hepatitis A vaccine with routine childhood vaccines at age twelve to fifteen months: a randomized controlled trial. Pediatr Infect Dis J. 2007; 26:787–793.







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download