INTRODUCTION
MATERIALS AND METHODS
Study subjects
Image acquisition
Scintigraphic diagnosis of RA
 | Fig. 1Findings are not compatible with rheumatoid arthritis (RA) on additional blood pool phase and bone phase of a patient with arthralgia. No significant increased blood pool activity (A) or periarticular bone uptake (B) in hands and wrists, elbows, feet, ankles and knees. |
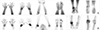 | Fig. 2Findings are compatible with rheumatoid arthritis (RA) on additional blood pool phase and bone phase of patients with RA. Increased blood pool activity (A) and periarticular bone uptake (B) are shown in right 2nd, 3rd, 5th, left 3rd, 5th PIP joints and left 1st IP joint of both hands, in both wrists, in right elbow, in right 4th, left 2nd, 3rd, and 5th MTP joints of both feet, in right ankle, and in right knee. |
Statistical analysis
RESULTS
Clinical and demographic features of the patients
 | Fig. 3Flowchart of the study design and number of patients enrolled. RA, rheumatoid arthritis; OA, osteoarthritis; PR, palindromic rheumatism; FMS, fibromyalgia syndrome; SpA, seronegative spondyloarthritis; AOSD, adult onset Still’s disease; Others, others not specified. |
Table 1
Baseline characteristics of the rheumatoid arthritis and non-rheumatoid arthritis groups with arthralgia
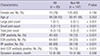
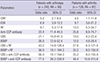
Scintigraphic diagnostic performance of BSBP and CBS for diagnosis of RA
Table 2
Comparison of diagnostic efficacies of conventional bone scintigraphy and bone scintigraphy with blood pool for diagnosis of rheumatoid arthritis

Odds ratio of BSBP and CBS for diagnosis of RA
Table 3
Value of conventional bone scintigraphy and bone scintigraphy with blood pool phase in predicting diagnosis of rheumatoid arthritis
Causes of false interpretations of BSBP and CBS
DISCUSSION
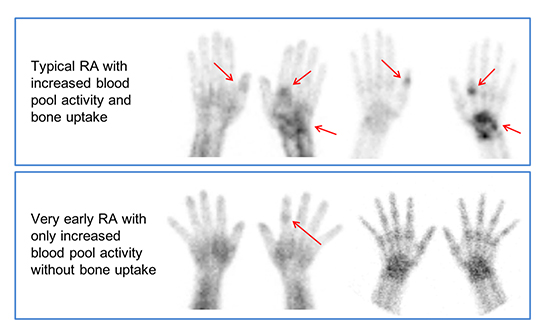
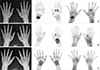 | Fig. 4Hand radiography and 99mTc-HDP bone scintigraphy findings in rheumatoid arthritis (RA) patients with positive RF and anti-CCP antibodies. (A) A 51-year old female patient with right wrist and bilateral hands pain for 10 years. Hand radiography (left column) shows uniform joint space narrowing with periarticular bony erosion in the right wrist and 1st, 2nd, and 3rd MCP joints of left hand which are suggestive of advanced rheumatoid arthritis. CBS (middle column) and blood pool image of BSBP (right column) show increased periarticular bone uptakes as well as fusiform increased blood pool activity in corresponding area of structural changes on radiography, finally interpreted as “compatible RA” on both CBS and BSBP. (B) A 45-year old female patient with left wrist, both hands and left ankle pain for 3 years. Hand radiography shows (left column) negative finding. However, CBS (middle column) and blood pool image of BSBP (right column) show increased periarticular bone uptakes as well as fusiform increased blood pool activity in right 1st IP, left 2nd MCP joins of both hands and left wrist, finally interpreted as “compatible RA” on both CBS and BSBP. (C) A 47 years old female patient with both hands and both feet pain for 1 month which might represent very early RA patient. Hand radiography (left column) and CBS (middle column) show negative finding. However, blood pool image of BSBP (right column) shows fusiform increased blood pool activity in 2nd PIP joint of left hand, finally interpreted as “compatible RA” only on BSBP. |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download