INTRODUCTION
Coagulation factor V (FV), known as labile factor or proaccelerin, plays a pivotal role in the blood coagulation cascade by having both procoagulant and anticoagulant functions (
1). FV is cofactor for the prothrombinase complex that activates prothrombin to thrombin and interacts with several coagulation factors and also modulator in the anticoagulant pathway by downregulating factor VIII activity. FV is mainly synthesized by liver and present mostly in plasma, accounting for approximately 80% of the circulating FV (
2). In addition to plasma FV, 20% of the total FV in whole blood is found within α-granules of platelets (
12). Although possibly megakaryocytes can synthesize FV, the majority of platelet FV originate from plasma through a mechanism of plasma FV uptake (endocytosis) by bone marrow megakaryocytes (
13). As consequence, the levels of FV are affected by liver synthetic function, and FV deficiency can lead to bleeding as a result of physiologic role of FV in contributing thrombin generation.
FV deficiency is a rare bleeding disorder (8.3% of all rare inherited bleeding disorders) with an estimated incidence of 1 in 1,000,000 (3-5), which is associated with a variable spectrum of bleeding manifestations ranging from mucosa and soft tissue bleeding (such as epistaxis and hemarthroses) to life-threatening hemorrhages. Until now, more than 200 cases of FV deficiency have been described in literature (
35). Inherited FV deficiency, also known as parahemophilia or Owen disease, had an autosomal recessive trait, and occurred approximately up to 10 times more frequently in West Asian countries where consanguineous marriage are common (such as Iran and southern India) than in the Western world (
4). In a North American registry for rare bleeding disorders published at 2004 (
6), while the majority of patients with FV deficiency were Caucasian accounting for 67% of all patients, Asian had only 2% of affected patients. Because of the relative rarity of FV deficiency in East Asia, there have been only a few case reports in Korea, and data on genetic, laboratory, and clinical characteristic of this disorder have been limited. Herein, we analyzed the clinical-laboratory characteristics and treatment outcomes in 10 Korean patients with FV deficiency. To our knowledge, this retrospective study is the first research for Korean patients with FV deficiency.
DISCUSSION
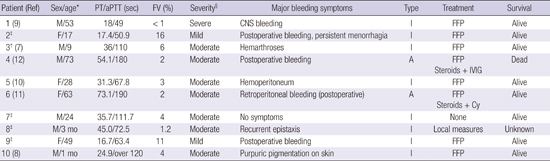
This is the first report to date of comprehensive analysis of FV deficiency in Korea, comprising eight inherited and two acquired FV-deficient patients. Whereas previous reports mainly focused on description of case series since 1987 when the first case was reported in Korea (
7), in the present study, we demonstrate the clinical manifestations, laboratory findings, and treatment outcomes in 10 Korean patients with FV deficiency. Inherited FV deficiency occurs with an estimated prevalence of one per million individuals worldwide (
3). Although no precise epidemiologic data existed for FV deficiency in Korea, only 10 patients were identified as having inherited or acquired FV deficiency during the study periods. However, because of lack of understanding about FV deficiency and ignorance of many minor bleeding episodes commonly by physicians, it is possible that the true incidence rate of FV deficiency in Korea is higher than expected. Due to rarity of this disorder, national comprehensive registry such as The North American registry for rare bleeding disorder in the USA (
6) or The Hemophilia Centre Directors Organization in the UK (
13) will be needed to gather data about disease prevalence, genotype information, clinical manifestations, treatment strategies and complications associated with disease or treatment.
In the literature, large series of FV deficiency usually involved both children and adults (
35614). In the Iranian cohort (
14), age distribution range was reported as 3 to 65 years and most patients had bleeding symptoms before the age of 6 years. The ages of our patients at diagnosis were ranging between 1 month and 73 years (median, 26 years). Two patients (20%) were diagnosed when they were younger than one year of age. Of the inherited FV-deficient patients in this study, only three had a positive family history for bleeding disorder, despite the autosomal recessive inheritance pattern for inherited FV deficiency.
FV deficiency may be presented with various bleeding patterns in affected patients; epistaxis, hemarthrosis, menorrhagia, gastrointestinal bleeding, hematuria, bleeding from umbilicus stump, and intracranial bleeding have been reported. Lak et al. (
14) in a series of 35 Iranian patients reported that epistaxis and oral cavity bleeding were the predominant symptoms (57%). In this study, most patients had variable bleeding events and predominantly involved mucous membranes of oropharyngeal space and genital area in females, which is generally similar to the findings in the Iranian study. In contrast, other symptoms of mucosal bleeding such as hematuria and melena were not reported. Of our cases, one was asymptomatic and diagnosis was made by routine laboratory work-up. In the Iranian cohort (
14), excessive post-operative bleeding (most often after circumcision) and CNS bleeding were presented in 43% and 6%, respectively. Additionally, Acharya et al. (
6) had reported the frequency of CNS bleeding as 8%. In our study, four patients presented with fatal bleeding symptoms and among them, one experienced overt intracranial hemorrhage. Another hemorrhage type in our study was postoperative bleeding with a frequency of 60%. Accordingly, this pattern of bleeding symptoms in our study is generally similar to that described by other studies.
The severity of bleeding symptoms of FV deficiency is variable and generally reported that the correlation between plasma FV levels and clinical manifestation is not clear-cut (
12). Many patients with undetectable levels of FV (< 1%) displayed only mild-to-moderate bleeding phenotype. Some patients with FV levels greater than 5% have severe bleeding symptom, even cases of the thrombosis have been reported (
2). Among the moderate to severe FV-deficient patients (n = 7) in our study, whereas four displayed fatal bleeding episodes, two had mild bleeding symptoms and one had no bleeding symptom. These observations of diverse clinical phenotype associated with FV levels strongly suggest the existence of additional factors modulating the bleeding diathesis in FV deficiency. As a possible explanation for these discrepancies, the recent studies identified the phenotypic modifiers of the bleeding predisposition in the severe FV-deficient patients, including platelet factor V, plasma tissue factor pathway inhibitor (TFPI), and pseudo-homozygous activated protein C resistance (
2). Currently, several studies show that residual platelet FV may be crucial in maintaining adequate hemostasis for patients with undetectable plasma FV levels (
12), where it might explain the discrepancy between FV levels and bleeding manifestations. In our study, platelet FV was not routinely evaluated, especially in severe FV-deficient patients, reporting only plasma FV levels, and we did not perform genotype and molecular analysis of FV molecules because of lack of specialized research laboratories to identify the molecular and/or genetic defects in patients with FV deficiency.
Because no FV concentrates are currently available, the mainstay of FV deficiency treatment in the presented study was infusion of FFP, which is widely available and relatively inexpensive (
23). FFP was used in 80% of patients, and, in most instances, they were given it before a diagnosis was confirmed. In our study, most congenital FV-deficient patients had a good treatment response for bleeding control and a favorable prognosis after single or repeated FFP administration, without any complications such as volume overload. In addition to replacement therapy, local measures or antifibrinolytic agents such as tranexamic acid alone or in combination with FFP administration may be useful in the management of the less severe bleeding cases (
4). Also combined oral contraceptives (COC) were used in two female patients with persistent menorrhagia to reduce menstrual blood loss and little response for bleeding control was observed.
Acquired FV deficiency associated with inhibitors is a rare event and may cause significant morbidity and mortality. Recently, about 150 cases of acquired FV deficiency, with only two Korean cases, have been reported in literatures (
15). In Korea, the first case was described in 2008 (
12). Variable risk factors previously reported to be associated with the occurrence of FV inhibitors included surgical procedures, antibiotics administration such as the beta lactams, blood transfusions, malignancy, autoimmune diseases, and exposure to bovine thrombin (
16). In our study, two patients had acquired FV deficiency with either idiopathic etiology or associated risk factors for the presence of FV inhibitors. Among them, one patient with acquired FV deficiency had proven inhibitors after cancer surgery, which may play a causative role in the development of the inhibitor. Also, the perioperative use of antibiotic (second-generation cephalosporin) and/or old age may be contributed to develop the FV inhibitors, being difficult to establish. Another patient with no definite risk factors, mentioned above, is described as idiopathic. The clinical phenotype of patients with acquired FV deficiency might vary from asymptomatic laboratory abnormalities to fatal bleeding (
1516). Our study showed that life-threatening bleedings including postoperative hemoperitoneum and retroperitoneal hemorrhage were observed in all patients with acquired FV inhibitors with one fatality among them, and both of them had severe FV deficiency with plasma FV levels of 2%. Ang et al. (
16) showed that in a retrospective review of 76 acquired FV-deficient patients with proven FV inhibitors, low FV levels (median 1%, range of 0%–23%,
P = 0.068) was associated with a tendency for the severe bleeding compared with non-bleeders, with no statistical significance. In our study, all acquired FV-deficient patients had plasma FV levels of 2%, and showed lethal bleeding events, which findings were consistent with previous studies.
Generally, the treatment strategy of acquired FV inhibitors included the bleeding control and the eradication of the FV inhibitors, especially in symptomatic patients (
1516). Variable hemostatic agents were used in attempts to control clinically severe bleeding events. They included FFP, platelets, prothrombin complex concentrate (PCC), recombinant activated factor VII (rVIIIa), with response rate of 15%, 69%, 80%, and 33%, respectively (
15). Especially, a consistent number of patients in published literatures had a significant clinical response (69%–71%) to transfusion of platelet concentrates which appear to be resistant to inhibitors until platelets are activated (
15). However, our patients with the inhibitors showed no response to repeated platelet transfusion and received no additional hemostatic agents such as PCC or rVIIa. In addition to the control of the bleeding, to eradicate the inhibitors and suppress production of the autoantibody, a number of therapeutic options including immunosuppressant, immunoadsorption, IVIG, and plasmapheresis have been used in bleeding patients with acquired FV inhibitor with varying success (
16). In a systemic review by Ang et al. (
16), 15 patients with FV autoantibodies were treated with steroids plus chemotherapy, including vincristine, cyclophosphamide, doxorubicin, and chlorambucil, with a response rate of 86.6%. Among two patients with FV inhibitor in our study, one of them developed massive retroperitoneal bleeding, and initially received FFP and platelet transfusion without response. Subsequently, immunosuppressive chemotherapy with high-dose steroids and cyclophosphamide was performed with the resolution of the FV activity on 4 weeks from start of treatment. However, the other patient who received corticosteroids and IVIG had no response with increased autoantibody titers and finally died of bleeding complications.
This study has potential limitations. Due to extreme rarity of the disease, limitations of this study were stemmed mostly from the small number of patients included and its retrospective nature, making it difficult for any solid conclusions to be drawn from these results. In addition, we could not analyse the genotype and molecule of FV in routine practice due to lack of specialized research laboratories to identify the molecular and/or genetic defects in FV-deficient patients. Despite of these inherent limitations, this study provides clinical data of Korean FV deficient-patients because FV deficiency is extremely rare disease and large clinical studies regarding this disease have not been reported in Korea.
In conclusion, despite its retrospective design and the small sample size, we found that Korean patients with FV deficiency had similar clinical manifestations and treatment outcomes shown in several previous studies. Mucosal tract bleedings were the predominant symptoms in FV deficient-patients and severity of bleeding symptoms were not always correlated with plasma FV levels. Most patients responded well in FFP replacement treatment, except the patients having an inhibitor to FV, who received an immunosuppressive therapy. Further studies with larger numbers of patients are warranted to define the actual incidence, clinical features, and appropriate therapeutic strategies and to clarify the accuracy of our results.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download