INTRODUCTION
CASE DESCRIPTION
 | Fig. 1Gross atrophy view of the patient. (A) Distal oblique portion of the left arm. (B) Proximal portion of the left arm. |
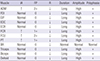
Table 1
Electromyography findings
Journal List > J Korean Med Sci > v.31(10) > 1023120

 | Fig. 1Gross atrophy view of the patient. (A) Distal oblique portion of the left arm. (B) Proximal portion of the left arm. |

Jinil Kim 
https://orcid.org/http://orcid.org/0000-0002-4706-7259
Yuntae Kim 
https://orcid.org/http://orcid.org/0000-0003-4063-4692
Sooa Kim 
https://orcid.org/http://orcid.org/0000-0003-1578-0452
Kiyoung Oh 
https://orcid.org/http://orcid.org/0000-0002-1886-5462