1. Parkinson D. A surgical approach to the cavernous portion of the carotid artery. Anatomical studies and case report. J Neurosurg. 1965; 23:474–483.
2. Dolenc VV. Anatomy and Surgery of the Cavernous Sinus. New York, NY: Springer-Verlag;1989.
3. Hakuba A, Tanaka K, Suzuki T, Nishimura S. A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989; 71:699–704.
4. van Loveren HR, Keller JT, el-Kalliny M, Scodary DJ, Tew JM Jr. The Dolenc technique for cavernous sinus exploration (cadaveric prosection). Technical note. J Neurosurg. 1991; 74:837–844.
5. Goel A. The extradural approach to lesions involving the cavernous sinus. Br J Neurosurg. 1997; 11:134–138.
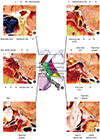
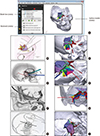
6. Isolan GR, Krayenbühl N, de Oliveira E, Al-Mefty O. Microsurgical anatomy of the cavernous sinus: measurements of the triangles in and around it. Skull Base. 2007; 17:357–367.
7. Dolenc VV, Rogers L. Cavernous Sinus: Developments and Future Perspectives. New York, NY: Springer;2009.
8. Cowan JA Jr, Ziewacz J, Dimick JB, Upchurch GR Jr, Thompson BG. Use of endovascular coil embolization and surgical clip occlusion for cerebral artery aneurysms. J Neurosurg. 2007; 107:530–535.
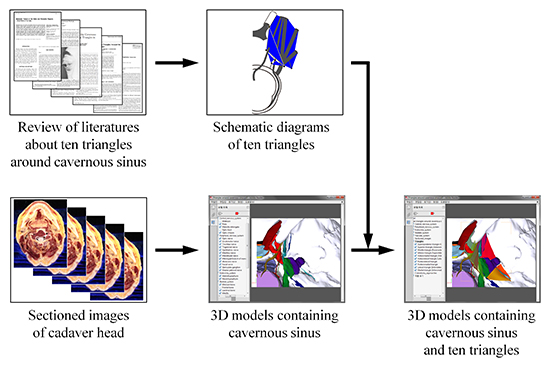
9. Park JS, Chung MS, Shin DS, Har DH, Cho ZH, Kim YB, Han JY, Chi JG. Sectioned images of the cadaver head including the brain and correspondences with ultrahigh field 70 T MRIs. Proc IEEE. 2009; 97:1988–1996.
10. Park HS, Chung MS, Shin DS, Jung YW, Park JS. Whole courses of the oculomotor, trochlear, and abducens nerves, identified in sectioned images and surface models. Anat Rec (Hoboken). 2015; 298:436–443.
11. Park JS, Chung MS, Hwang SB, Lee YS, Har DH. Technical report on semiautomatic segmentation using the Adobe Photoshop. J Digit Imaging. 2005; 18:333–343.
12. Park JS, Chung MS, Chi JG, Park HS, Shin DS. Segmentation of cerebral gyri in the sectioned images by referring to volume model. J Korean Med Sci. 2010; 25:1710–1715.
13. Shin DS, Chung MS, Park JS, Park HS, Lee S, Moon YL, Jang HG. Portable document format file showing the surface models of cadaver whole body. J Korean Med Sci. 2012; 27:849–856.
14. Shin DS, Lee S, Park HS, Lee SB, Chung MS. Segmentation and surface reconstruction of a cadaver heart on Mimics software. Folia Morphol (Warsz). 2015; 74:372–377.
15. Park JS, Chung MS, Park HS, Shin DS, Har DH, Cho ZH, Kim YB, Han JY, Chi JG. A proposal of new reference system for the standard axial, sagittal, coronal planes of brain based on the serially-sectioned images. J Korean Med Sci. 2010; 25:135–141.
16. Mizoi K, Suzuki J, Kinjo T, Yoshimoto T. Bifrontal interhemispheric approach for carotid-ophthalmic aneurysms. Acta Neurochir (Wien). 1988; 90:84–90.
17. Curey S, Derrey S, Hannequin P, Hannequin D, Fréger P, Muraine M, Castel H, Proust F. Validation of the superior interhemispheric approach for tuberculum sellae meningioma: clinical article. J Neurosurg. 2012; 117:1013–1021.
18. Gonzalez LF, Amin-Hanjani S, Bambakidis NC, Spetzler RF. Skull base approaches to the basilar artery. Neurosurg Focus. 2005; 19:E3.
19. Kim JS, Lee SI, Jeon KD, Choi BS. The pterional approach and extradural anterior clinoidectomy to clip paraclinoid aneurysms. J Cerebrovasc Endovasc Neurosurg. 2013; 15:260–266.
20. Gonzalez FL, Ferreira MA, Zabramski JM, Spetzler RF, Deshmukh P. The middle fossa approach. BNI Q. 2000; 16:1–4.
21. Schmidek HH, Sweet WH. Operative Neurosurgical Techniques: Indications, Methods and Results. 4th ed. Philadelphia, PA: W.B. Saunders;2000.
22. Fujimoto Y, Ikeda H, Yamamoto S. Pterional transcavernous approach for large basilar top aneurysm: significance of the exposure of Dolenc's triangle. Surg Cereb Stroke. 1992; 20:191–195.
23. Watanabe A, Nagaseki Y, Ohkubo S, Ohhashi Y, Horikoshi T, Nishigaya K, Nukui H. Anatomical variations of the ten triangles around the cavernous sinus. Clin Anat. 2003; 16:9–14.
24. Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996; 38:425–432.
25. Federative Committee on Anatomical Terminology. Terminologia Anatomica: International Anatomical Terminology. Stuttgart: Thieme;1998.
26. Yoon BH, Kim HK, Park MS, Kim SM, Chung SY, Lanzino G. Meningeal layers around anterior clinoid process as a delicate area in extradural anterior clinoidectomy : anatomical and clinical study. J Korean Neurosurg Soc. 2012; 52:391–395.
27. Abla AA, Lawton MT. Current treatment strategies for cavernous internal carotid artery aneurysms. World Neurosurg. 2014; 82:994–995.
28. Tripathi M, Deo RC, Suri A, Srivastav V, Baby B, Kumar S, Kalra P, Banerjee S, Prasad S, Paul K, et al. Quantitative analysis of the Kawase versus the modified Dolenc-Kawase approach for middle cranial fossa lesions with variable anteroposterior extension. J Neurosurg. 2015; 123:14–22.
29. Lunsford LD. Contemporary management of meningiomas: radiation therapy as an adjuvant and radiosurgery as an alternative to surgical removal? J Neurosurg. 1994; 80:187–190.
30. Schreuder HW, Pruszczynski M, Veth RP, Lemmens JA. Treatment of benign and low-grade malignant intramedullary chondroid tumours with curettage and cryosurgery. Eur J Surg Oncol. 1998; 24:120–126.
31. Yi HJ, Kim CH, Bak KH, Kim JM, Ko Y, Oh SJ. Metastatic tumors in the sellar and parasellar regions: clinical review of four cases. J Korean Med Sci. 2000; 15:363–367.
32. Myrseth E, Møller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma: surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009; 64:654–661.
33. Ammirati M, Wei L, Ciric I. Short-term outcome of endoscopic versus microscopic pituitary adenoma surgery: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2013; 84:843–849.
34. Nichols DA, Brown RD Jr, Meyer FB. Coils or clips in subarachnoid haemorrhage? Lancet. 2002; 360:1262–1263.
35. Byrne JV. The aneurysm “clip or coil” debate. Acta Neurochir (Wien). 2006; 148:115–120.
36. Debrun GM, Nauta HJ, Miller NR, Drake CG, Heros RC, Ahn HS. Combining the detachable balloon technique and surgery in imaging carotid cavernous fistulae. Surg Neurol. 1989; 32:3–10.








 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download