INTRODUCTION
MATERIALS AND METHODS
Mice
Study flow
 | Fig. 1Experimental protocol of our study.
Female C57BL/6 mice were treated with intratracheal instillation of bleomycin (BLM) or saline on day 0. Metformin (50 mg/kg or 100 mg/kg, according to group assignment) or PBS was administered orally once a day from day 1 onward. Half the mice were sacrificed on day 10 and the remaining mice were sacrificed on day 21.
|
Metformin treatment
Generation of a murine BLM-induced lung fibrosis model
Analysis of bronchoalveolar lavage fluid
Histologic analysis
Isolation of RNA and real-time polymerase chain reaction (RT-PCR)
RESULTS
Metformin reduced BLM-induced recruitment of inflammatory cells in the lungs
 | Fig. 2Effect of metformin treatment on the total and differential cell counts in the bronchoalveolar fluid.
The total and differential cell counts in the bronchoalveolar fluid were determined on days 10 and 21 after the instillation of bleomycin (BLM). Metformin (MFM) was administered orally to mice once a day from day 1 to either day 10 or 21. Data are shown as mean ± SEM. *
P < 0.05.
(A) Total cell count on day 10. (B) Number of inflammatory cells on day 10. (C) Total cell count on day 21. (D) Number of inflammatory cells on day 21. Day 10: control, MFM, n = 5; BLM, BLM + MFM 50 mg/kg, n = 7; BLM + MFM 100 mg/kg, n = 6 (1 dead). Day 21: control, MFM-only, n = 5; BLM-only, n = 2 (5 dead); BLM + MFM 50 mg/kg, n = 4 (3 dead); BLM + MFM 100 mg/kg, n = 6 (1 dead).
|
Metformin decreased BLM-induced inflammation, fibrosis, and collagen deposition in lung tissue
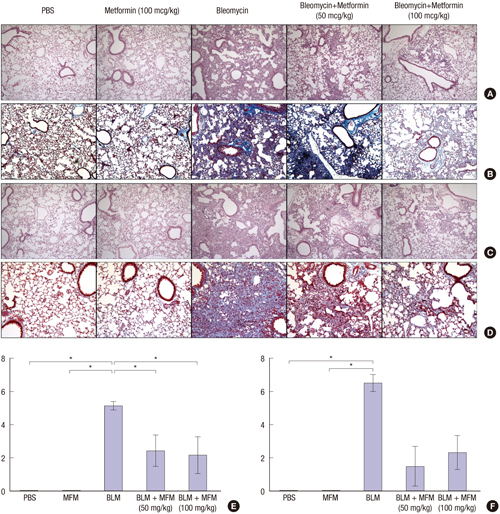
 | Fig. 3Effect of metformin on the infiltration of inflammatory cells and collagen deposition in the lungs of BLM-treated mice.
(A) Histologic findings with hematoxylin & eosin (H & E) staining on day 10. (B) Histologic findings with Masson’s trichrome staining on day 10. (C) Histologic findings with H & E staining on day 21. (D) Histologic findings with Masson’s trichrome staining on day 21. (E) Ashcroft fibrosis scores on day 10. (F) Ashcroft fibrosis score on day 21. Representative magnification, × 200.
|
Measurement of mRNA levels in the lungs
 | Fig. 4mRNA levels of collagen, collagen-1, procollagen, fibronectin, and TGF-β in lung tissue on day 21.
(A) Collagen, (B) collagen-1, (C) procollagen, (D) fibronectin, (E) TGF-β. Day 21: control, MFM, n = 5; BLM, n = 2 (5 dead); BLM + MFM 50 mg/kg, n = 4 (3 dead); BLM + MFM 100 mg/kg, n = 6 (1 dead). *P < 0.05.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download