1. Consensus development conference on the diagnosis of coronary heart disease in people with diabetes: 10-11 February 1998, Miami, Florida. American Diabetes Association. Diabetes Care. 1998; 21:1551–1559.
2. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998; 339:229–234.
3. Cheung N, Wang JJ, Klein R, Couper DJ, Sharrett AR, Wong TY. Diabetic retinopathy and the risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2007; 30:1742–1746.
4. Rosenson RS, Fioretto P, Dodson PM. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis. 2011; 218:13–18.
5. Targher G, Bertolini L, Zenari L, Lippi G, Pichiri I, Zoppini G, Muggeo M, Arcaro G. Diabetic retinopathy is associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabet Med. 2008; 25:45–50.
6. Tsujimoto T, Kajio H, Takahashi Y, Kishimoto M, Noto H, Yamamoto-Honda R, Kamimura M, Morooka M, Kubota K, Shimbo T, et al. Asymptomatic coronary heart disease in patients with type 2 diabetes with vascular complications: a cross-sectional study. BMJ Open. 2011; 1:e000139.
7. Yoon KC, Mun GH, Kim SD, Kim SH, Kim CY, Park KH, Park YJ, Baek SH, Song SJ, Shin JP, et al. Prevalence of eye diseases in South Korea: data from the Korea National Health and Nutrition Examination Survey 2008-2009. Korean J Ophthalmol. 2011; 25:421–433.
8. Kawasaki R, Cheung N, Islam FM, Klein R, Klein BE, Cotch MF, Sharrett AR, O’Leary D, Wong TY; Multi-Ethnic Study of Atherosclerosis. Is diabetic retinopathy related to subclinical cardiovascular disease? Ophthalmology. 2011; 118:860–865.
9. Koistinen MJ. Prevalence of asymptomatic myocardial ischaemia in diabetic subjects. BMJ. 1990; 301:92–95.
10. Ditchburn CJ, Hall JA, de Belder M, Davies A, Kelly W, Bilous R. Silent myocardial ischaemia in patients with proved coronary artery disease: a comparison of diabetic and non-diabetic patients. Postgrad Med J. 2001; 77:395–398.
11. Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006; 141:446–455.
12. Klein R, Sharrett AR, Klein BE, Moss SE, Folsom AR, Wong TY, Brancati FL, Hubbard LD, Couper D. ARIC Group. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes : the atherosclerosis risk in communities study. Ophthalmology. 2002; 109:1225–1234.
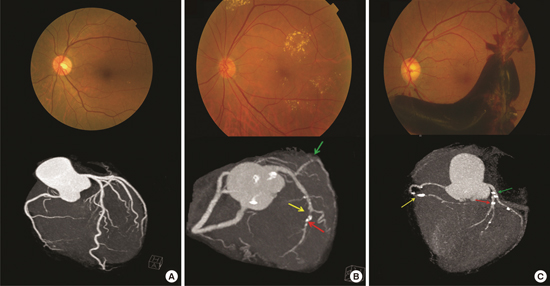
13. Rong J, Yu CQ, Yang P, Chen J. Association of retinopathy with coronary atherosclerosis determined by coronary 64-slice multidetector computed tomography angiography in type 2 diabetes. Diab Vasc Dis Res. 2013; 10:161–168.
14. Johnson TR, Nikolaou K, Wintersperger BJ, Leber AW, von Ziegler F, Rist C, Buhmann S, Knez A, Reiser MF, Becker CR. Dual-source CT cardiac imaging: initial experience. Eur Radiol. 2006; 16:1409–1415.
15. Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008; 358:1336–1345.
16. American Diabetes Association. Nutrition recommendations and principles for people with diabetes mellitus. Diabetes Care. 2000; 23:Suppl 1. S43–6.
17. Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989; 321:129–135.
18. Puel J, Valensi P, Vanzetto G, Lassmann-Vague V, Monin JL, Moulin P, Ziccarelli C, Mayaudon H, Ovize M, Bernard S, et al. Identification of myocardial ischemia in the diabetic patient. Joint ALFEDIAM and SFC recommendations. Diabetes Metab. 2004; 30:3S3–3S18.
19. Rydén L, Standl E, Bartnik M, Van den Berghe G, Betteridge J, de Boer MJ, Cosentino F, Jönsson B, Laakso M, Malmberg K, et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur Heart J. 2007; 28:88–136.
20. Cosson E, Nguyen MT, Chanu B, Balta S, Takbou K, Valensi P. The report of male gender and retinopathy status improves the current consensus guidelines for the screening of myocardial ischemia in asymptomatic type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2013; 23:557–565.










 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download