INTRODUCTION
CASE DESCRIPTION
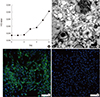
Fig. 1

Fig. 2
Journal List > J Korean Med Sci > v.31(7) > 1023035


Funding Eun Hwa Choi and Nam-Joong Kim are participating investigators of the research program funded by the Korea Centers for Disease Control and Prevention (2016P2400100).
AUTHOR CONTRIBUTION Patient care: Jang HC, Kim UJ, Jung SI. Isolation of virus: Jang HC, Park WB, Kim UJ, Chun JY, Choi SJ, Choe PG, Oh MD. RT-PCR for virus: Jee Y. Study design and concept: Jang HC, Choi EH, Oh MD. Reference review: Jang HC, Park WB, Kim UJ, Oh MD. Writing: Jang HC, Park WB, Kim UJ, Oh MD. Critical review and revision: Jang HC, Park WB, Jung SI, Kim NJ, Oh MD.
Hee-Chang Jang 
https://orcid.org/http://orcid.org/0000-0002-3407-8493
Wan Beom Park 
https://orcid.org/http://orcid.org/0000-0003-0022-9625
Uh Jin Kim 
https://orcid.org/http://orcid.org/0000-0002-8463-6297
June Young Chun 
https://orcid.org/http://orcid.org/0000-0001-9345-6645
Su-Jin Choi 
https://orcid.org/http://orcid.org/0000-0001-8732-3950
Pyoeng Gyun Choe 
https://orcid.org/http://orcid.org/0000-0001-6794-7918
Sook-In Jung 
https://orcid.org/http://orcid.org/0000-0002-1577-678X
Nam-Joong Kim 
https://orcid.org/http://orcid.org/0000-0001-6793-9467
Eun Hwa Choi 
https://orcid.org/http://orcid.org/0000-0002-5857-0749
Myoung-don Oh 
https://orcid.org/http://orcid.org/0000-0002-2344-7695