Abstract
Graphical Abstract
Figures and Tables
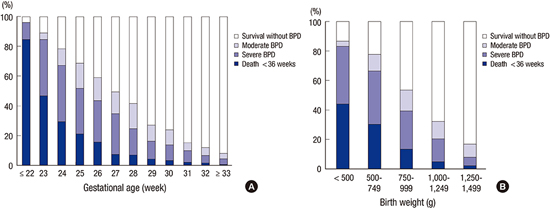
Fig. 1
Three possible outcomes (death before 36 weeks PMA, survival with BPD or survival without BPD) of 2,386 VLBW infants at 36 weeks PMA, which are sub-grouped according to the (A) gestational age (by 1 week) and (B) birth weight (by 250 grams). PMA, postmenstrual age; BPD, bronchopulmonary dysplasia; VLBW, very-low-birth-weight.

Fig. 2
Changes of the rate of death before 36 weeks, survival with BPD or survival without BPD at 36 weeks PMA from 2013-mid 2014 compared to 2007-2008 (10) among VLBW infants born at 23-31 weeks of gestation. In total, 1,990 VLBW infants were compared to 3,841 VLBW infants from the nationwide survey in 2007-2008 (10). The distribution of the overall rate of the three possible outcomes at 36 weeks PMA were different compared to that in the survey from 2007-2008 (10) using the chi-square test (P < 0.001). The incidence of BPD increased by 85% (from 17.8% to 33.0%); the rate of severe BPD increased by 157% (from 8.1% to 20.8%); the rate of death before 36 weeks' PMA decreased by 28.9% (from 15.9% to 11.3%) compared to the retrospective survey in 2007-2008 (10). BPD, bronchopulmonary dysplasia; PMA, postmenstrual age; VLBW, very-low-birth-weight.

Table 1
Perinatal and initial clinical characteristics of bronchopulmonary dysplasia among the surviving 2,136 very-low-birth-weight infants at 36 weeks postmenstrual age
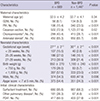
*Pathologic chorioamnionitis; †completed cases of antenatal steroid; ‡pulmonary hemorrhage, air leak, and pulmonary hypertension were included; §limited to hemodynamically significant cases which require medical or surgical treatment. BPD, bronchopulmonary dysplasia; GDM, gestational diabetes mellitus; PIH, pregnancy induced hypertension; PROM, premature rupture of membrane; PDA, patent ductus arteriosus.
Table 2
Effects of associated perinatal variables on bronchopulmonary dysplasia using multivariate logistic regression analysis among the surviving 2,136 very-low-birth-weight infants at 36 weeks postmenstrual age
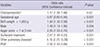
Table 3
Clinical outcomes and treatment characteristics of bronchopulmonary dysplasia among the surviving 2,136 very-low-birth-weight infants at 36 weeks postmenstrual age and the effects of associated variables on bronchopulmonary dysplasia

Table 4
Comparison of outcomes at 36 weeks postmenstrual age among very-low-birth-weight infants from 2007-2008 to 2013-mid-2014 according to the birth weight groups

A total of 1,990 VLBW infants born at 23-31 weeks of gestation were compared to 3,841 VLBW infants from the nationwide survey in 2007-2008 (10). The published results and partial available raw data from the survey (10) were used. The distribution of the three possible outcomes at 36 weeks PMA were different compared to that from 2007-2008 (10) using the chi-square test (P<0.001). VLBW, very-low-birth-weight; Death, death before 36 weeks of postmenstrual age; BPD, bronchopulmonary dysplasia; Non-BPD, survival infants without BPD at 36 weeks of postmenstrual age.
Notes
Funding This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2013-E63008-01).
AUTHOR CONTRIBUTION Conception and design of the study: Jo HS, Cho SI, Cho KH, Song ES, Kim BI. Acquisition of data: Jo HS, Cho SI, Cho KH, Statistical Analysis: Cho SI, Cho KH. First draft of the manuscript: Jo HS, Cho KH. Revision and critical review of the manuscript: Jo HS, Cho SI, Cho KH, Song ES, Kim BI. Manuscript approval: all authors.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download