Abstract
Graphical Abstract

Figures and Tables
Fig. 1
Diagram of the launching and establishment of the Korean Neonatal Network (KNN). For the launching of the KNN, various activities, including planning, preparation, the actual process, structuring, and organization of the KNN were conducted since August 2012. Finally, KNN was officially started on April 15, 2013. TFT, task force team; CDC, Centers for Disease Control and Prevention; KSN, Korean Society of Neonatology; NRN, Neonatal Research Network.

Fig. 2
Organizational structure and committee members of the Korean Neonatal Network (KNN) at present (April 2015). Under the supervision and support from the Korea CDC, KNN is composed of the executive and advisory committees. The executive committee comprises an executive director (Dr. Won Soon Park), an executive secretary general (Dr. Yun Sil Chang), 12 executive board members, and 10 sub-executive board members, along with a central coordinator, and data managers and monitors. The following are the four subcommittees in the KNN: 1) the protocol subcommittee comprised of short-term (Dr. Byung Min Choi as a sub-director) and long-term divisions (Dr. Ellen Ai-Rhan Kim as a sub-director), 2) data and monitoring subcommittee (Dr. Yun Sil Chang as a sub-director), 3) education subcommittee (Dr. Eun Ae Park as a sub-director), and 4) ethics and publication subcommittee (Dr. So Young Kim as a sub-director). The advisory committee comprises mainly the steering members of the Korean Society of Neonatology (KSN), including Dr. Chong Woo Bae (the former president of KSN), Dr. Rhan Namgung (the president of KSN), Dr. Beyong Il Kim (the vice president of KSN), Dr. Ki Soo Kim (the vice president of KSN), Dr. Soo Chul Cho (the vice president of KSN), and others. *Symbol of Korean Neonatal Network. KNN, Korean Neonatal Network; CDC, Centers for Disease Control and Prevention; PI, principal investigator.

Fig. 3
The unique systems specifically developed in the Korean Neonatal Network (KNN). KNN established unique systems, including not only organization structure and Web-based e-CRF registry but also a real-time data display system on the Website, a data-quality maintenance system using query generation and site-visit monitoring, and a feedback system using annual reports. The KNN consists of well-structured systems for education for registries and studies, as well as the online application of data use and study proposal. e-CRF, electronic case report form; iCReaT, internet-based clinical research and trial; CDC, Centers for Disease Control and Prevention; ID, identification; PI, principal investigator; Q&A, question and answer.

Fig. 4
Diagram of the participation process in the Korean Neonatal Network (KNN). When the principal investigator (PI) in a certain hospital applies to participate in the KNN, the PI has to sign the pledge first for fulfilling the obligations and commitments, keeping the information secure during KNN participation. Then, the PI has to obtain IRB approval for the KNN registry from his/her hospital and required official education for the use of the iCReaT. When the PI submit IRB approval document to the KNN, the iCReat ID that enables access to the KNN Web-based registry was provided. After receiving the KNN participation certificate plate, the institution can start the KNN registry. To view their own real-time data display on the secure KNN member Web site, PIs have to confirm the entered data to the KNN e-CRF and resolve the auto and manual queries sent from the KNN center periodically. In addition, the PI has to accept the KNN site-visit monitoring twice a year for quality surveillance of data and the KNN administrative process. Accumulated data are locked periodically, followed by cleaning and statistical analysis by central data managers. Then, the KNN executive committee publishes a total or individual annual report of the previous year. It provides powerful interactive data-driven evidence for quality improvement to the individual participating center. Academic studies that used the KNN data are promoted through the study proposal application system. Active collaborations among participants occur directly in the education or PI seminar once a year and indirectly but continuously through the KNN Web site. PI, principal investigator; IRB, institutional review board; iCReaT, internet-based clinical research and trial; ID, identification.
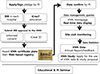
Fig. 5
A diagrams included in the individual and confidential annual report of the KNN, showing the odds ratios (ORs) and 95% confidence intervals (CIs) for various treatment policies and outcomes, with adjustment for the gestational age of the enrolled VLBWIs. This diagram shows that Center 70474 (virtual center, not real) has significantly lower incidences of moderate to severe BPD and postnatal corticosteroid use than all the other participating hospitals, with adjusted OR of 0.5 and 0.3, respectively. These data-driven analyses provide a valuable and powerful feedback for quality improvement to each institution, where the institution positions among all of the KNN participating institutions in terms of specific items indicating care qualities.

Fig. 6
KNN Study site, an electronic application system for study proposal and data use on the Members site of KNN homepage (http://knn.or.kr). Any investigators of the participating hospitals, which registered more than 10 cases to the KNN, have the right to apply the study proposal by using this system at any time. KNN, Korean Neonatal Network.
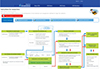
Table 1
Major international neonatal networks

| Name | Country | Year | Participating units | Registry inclusion at birth |
|---|---|---|---|---|
| NICHD Neonatal Research Network14) | United States | 1986~ | 21 tertiary care center | - <29 w or 401-1,000 g |
| - Follow-up for <27 w | ||||
| Vermont Oxford Neonatal Network (VON)15) | United States, Asia, Europe, Africa | 1988~ | 900 units | - 401-1,500 g or 22 w-29 w |
| - Others | ||||
| Australian and New Zealand Neonatal Network (ANZNN)16) | Australia, New Zealand | 1994~ | 29 level 3 units | - < 32 w or < 1,500 g or received assisted ventilation ≥ 4 hr, or major or therapeutic hypothermia |
| 26 level 2 units | ||||
| Canadian Neonatal Network (CNN)17) | Canada | 1995~ | 30 units from | - Total admitted |
| 17 universities | - <33 w or <1,500 g, HIE | |||
| Neonatal Research Network Japan (NRNJ)18) | Japan | 2003~ | 171 units | - <1,500g |
| - Hypothermia registry | ||||
| - Clinical trials | ||||
| National Neonatal Audit Programme (NNAP)19) | England | 2006~ | 179 units | - Various audit questions for < 32 w or < 1,501 g, or others |
| EuroNeoNet20) | 16 countries in Europe | 2006~ | 60 NICUs | - < 1,501 g or < 32 w |
| Korean Neonatal Network (KNN)23) | The Republic of Korea | 2013~ | 60 NICUs | - < 1,500 g |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download