1. Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31:143–178.
2. Azuma A, Kudoh S. Diffuse panbronchiolitis in East Asia. Respirology. 2006; 11:249–261.
3. Tsang KW, Ooi CG, Ip MS, Lam WK, Ngan H, Chan EY, Hawkins B, Ho CS, Amitani R, Tanaka E, et al. Clinical profiles of Chinese patients with diffuse panbronchiolitis. Thorax. 1998; 53:274–280.
4. Matsuno O, Ueno K, Hayama Y, Honda H, Yamane H, Saeki Y. Deterioration of asthma in a patient with diffuse panbronchiolitis (DPB) after macrolide therapy. J Asthma. 2010; 47:486–488.
5. Kadota J, Mukae H, Ishii H, Nagata T, Kaida H, Tomono K, Kohno S. Long-term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respir Med. 2003; 97:844–850.
6. Poletti V, Casoni G, Chilosi M, Zompatori M. Diffuse panbronchiolitis. Eur Respir J. 2006; 28:862–871.
7. Schultz MJ. Macrolide activities beyond their antimicrobial effects: macrolides in diffuse panbronchiolitis and cystic fibrosis. J Antimicrob Chemother. 2004; 54:21–28.
8. Park HY, Suh GY, Chung MP, Kim H, Kwon OJ, Chung MJ, Kim TS, Lee KS, Koh WJ. Comparison of clinical and radiographic characteristics between nodular bronchiectatic form of nontuberculous mycobacterial lung disease and diffuse panbronchiolitis. J Korean Med Sci. 2009; 24:427–432.
9. Kudoh S, Azuma A, Yamamoto M, Izumi T, Ando M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am J Respir Crit Care Med. 1998; 157:1829–1832.
10. Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin Microbiol Rev. 2010; 23:590–615.
11. Fujii T, Kadota J, Kawakami K, Iida K, Shirai R, Kaseda M, Kawamoto S, Kohno S. Long term effect of erythromycin therapy in patients with chronic Pseudomonas aeruginosa infection. Thorax. 1995; 50:1246–1252.
12. Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007; 357:1487–1495.
13. Marri PR, Stern DA, Wright AL, Billheimer D, Martinez FD. Asthma-associated differences in microbial composition of induced sputum. J Allergy Clin Immunol. 2013; 131:346–352.e1-3.
14. Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:2355–2365.
15. Wenzel RP, Fowler AA 3rd, Edmond MB. Antibiotic prevention of acute exacerbations of COPD. N Engl J Med. 2012; 367:340–347.
16. Wallwork B, Coman W, Mackay-Sim A, Greiff L, Cervin A. A double-blind, randomized, placebo-controlled trial of macrolide in the treatment of chronic rhinosinusitis. Laryngoscope. 2006; 116:189–193.
17. Hansen CR, Pressler T, Hoiby N, Johansen HK. Long-term, low-dose azithromycin treatment reduces the incidence but increases macrolide resistance in Staphylococcus aureus in Danish CF patients. J Cyst Fibros. 2009; 8:58–62.
18. Phaff SJ, Tiddens HA, Verbrugh HA, Ott A. Macrolide resistance of Staphylococcus aureus and Haemophilus species associated with long-term azithromycin use in cystic fibrosis. J Antimicrob Chemother. 2006; 57:741–746.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



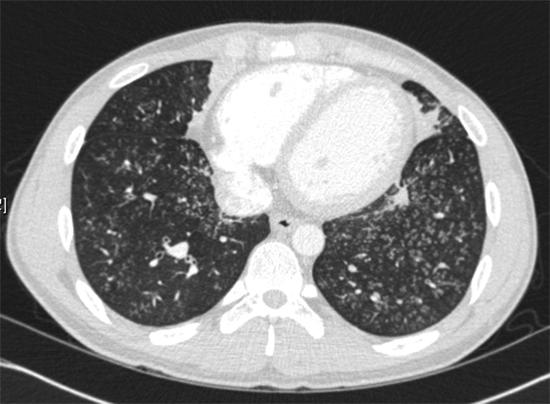

 XML Download
XML Download