Abstract
History of treatment for tuberculosis (TB) is a risk factor for obstructive lung disease. However, it has been unclear whether the clinical characteristics of patients with destroyed lung by TB differ according to the presence or absence of airflow limitation. The objective of the study was to evaluate differences in acute exacerbations and forced expiratory volume in 1 second (FEV1) decline in patients with destroyed lung by TB according to the presence or absence of airflow limitation. We performed a retrospective cohort study and enrolled patients with destroyed lung by TB. The presence of airflow limitation was defined as FEV1/forced vital capacity (FVC) < 0.7. One hundred and fifty-nine patients were enrolled, and 128 (80.5%) had airflow limitation. The proportion of patients who experienced acute exacerbation was higher in patients with airflow limitation compared to those without (89.1 vs. 67.7%, respectively; P = 0.009). The rate of acute exacerbation was higher in patients with airflow limitation (IRR, 1.19; 95% CI, 1.11-1.27). Low body mass index (X vs. X + 1; HR, 0.944; 95% CI, 0.895-0.996) in addition to airflow limitation (HR, 1.634; 95% CI, 1.012-2.638), was an independent risk factor for acute exacerbation. The annual decline of FEV1 was 2 mL in patients with airflow limitation and 36 mL in those without (P < 0.001). In conclusion, the presence of airflow limitation is an independent risk factor for acute exacerbation in patients with the destroyed lung by TB.
Tuberculosis (TB) is the second most common cause of death due to infectious disease globally (1). It can cause pulmonary sequelae after microbiological cure; these are characterized by bronchial and parenchymal destruction, including bronchovascular distortion, bronchiectasis, emphysematous changes, and fibrotic bands (2). These changes, which are collectively called destroyed lung by TB, result in respiratory functional disability (3).
The pulmonary physiologic sequelae in these patients are obstructive, restrictive, or mixed changes. The prevalence of each abnormality varies among reports. Plit et al. (3) reported residual airflow limitation or a restrictive pattern in 28% and 24% of patients, respectively. By contrast, Chae et al. (4) reported obstructive ventilatory defects, including mixed defects, in 86.4% of patients with destroyed lung by TB.
Although the prevalence of airflow limitation in patients with destroyed lung by TB differs markedly (3, 4, 5), destroyed lung by TB is a risk factor for obstructive lung disease (5, 6). The possible etiology of airflow limitation in these patients is either smoking itself (7) or peripheral airway collapse and subsequent air trapping due to parenchymal destruction (8).
Considering the similar physiological abnormalities, the clinical manifestations of patients with destroyed lung by TB with airflow limitation could be similar to those of patients with chronic obstructive pulmonary disease (COPD). A few studies have compared the clinical characteristics of those two groups of patients. Lee and Chang (9) reported that a positive bronchodilator response was observed more frequently in patients with COPD than in patients with chronic airflow obstruction secondary to TB. Another study reported that patients with destroyed lung by TB showed more diminished pulmonary function and needed more tracheostomies than did patients with COPD in intensive care unit setting (10).
In a substantial number of patients with destroyed lung by TB, pulmonary physiological defects were observed to be purely obstructive changes. Some previous studies have shown both similarities and differences in the clinical manifestations of patients with destroyed lung by TB and airflow limitation and those with COPD. However, whether the clinical course and long-term outcomes of patients with destroyed lung by TB differ according to the presence or absence of airflow limitation remains unclear. The objective of the present study was to evaluate the impact of airflow limitation on the rate of acute exacerbation and forced expiratory volume in 1-second (FEV1) decline in patients with destroyed lung by TB.
We enrolled patients with pulmonary TB who were followed up at Seoul National University Hospital from January, 2005 to December, 2011. Destroyed lung by TB was defined as follows: previous history of antituberculosis treatment, negative acid-fast bacilli smear and mycobacterial culture for tuberculosis on the sputum sample, and parenchymal destruction in greater than 25% of a hemithorax on chest radiograph. Patients who were followed up for more than 3 yr and who had more than two spirometries during the followed-up period were enrolled. The following patients were excluded from the study: those with active TB, nontuberculous mycobacteria infection, or other lung disease-such as pneumoconiosis, idiopathic fibrosis, and lung cancer. Patients with a history of lung resectional surgery were also excluded.
We performed a retrospective cohort study. Demographic, radiologic, and clinical parameters were compared between patients with and without airflow limitation.
The presence of airflow limitation was defined as FEV1/forced vital capacity (FVC) less than 70%. Extent of destruction was graded as three categories. If the extent of destruction on chest radiograph was less than one-third of the hemithorax, it was classified as grade 1. If the extent of destruction was between one- and two-thirds, it was classified as grade 2. If the extent of destruction was more than two-thirds, it was classified as grade 3.
Acute exacerbation was defined as an increase in or new onset of more than one respiratory symptom (cough, sputum, or dyspnea) requiring a prescription of antibiotics, systemic steroid, or both or hospitalization. Frequent exacerbators were defined as patients who had two or more acute exacerbations per year. All exacerbations were separated by ≥1 month.
Differences in parameters between two groups were evaluated using the chi-square test or Student's t-test. The rate of acute exacerbation was assessed using a Poisson regression model. Independent risk factors for exacerbation (with their 95% confidences interval [CIs]) were estimated by Cox regression analysis, including BMI as a continuous variable. Lung function decline was assessed using a mixed linear regression model.
In total, 386 patients were screened, and 159 were enrolled (Fig. 1). The baseline characteristics, lung function, and extent of destruction are shown in Tables 1 and 2.
Among the 159 patients, 128 (80.5%) had airflow limitation at the time of diagnosis of destroyed lung by TB. In patients with airflow limitation, 60 patients were classified pure obstructive pattern without total lung capacity (TLC). Of the remaining 68 patients, TLC was estimated in 12 patients. Four patients had obstructive pattern and 8 patents had mixed pattern. In patients without airflow limitation, 26 patients showed restrictive pattern on spirometry and 5 patients had normal spirometry. The baseline characteristics of patients with or without airflow limitation were not significantly different, except for a higher body mass index (BMI) in patients with air flow limitation (21.83±3.69 vs. 19.94±4.50; P=0.016) (Table 3).
Baseline lung function was evaluated. The % predicted value, but not absolute value, of FVC was significantly higher, and both the % predicted and absolute values of FEV1 was significantly lower in patients with airflow limitation compared to those without. When subgroup analysis was performed between 64 patients with pure obstructive pattern and 8 patients with mixed pattern, FEV1 as well as FVC were higher in patients with pure obstructive pattern whether expressed as absolute (1.26±0.48 vs. 0.90±0.17 L, P<0.001; 2.68±0.82 vs. 1.88±0.32 L, P<0.001, respectively) or predicted values (51.73±18.38 vs. 32.75%± 8.63%, P<0.001; 75.77±15.11 vs. 51.13%±8.71%, P<0.001, respectively). There was no significant difference in the extent of destruction between the two groups (Table 4).
The proportion of patients who experienced acute exacerbation at least once during the period was higher in patients with airflow limitation compared to those without (89.1 vs. 67.7%; P= 0.009) (Table 5). The duration of follow up was not significantly different between the two groups (Table 4). When only 55 never smokers with airflow limitation were compared with those without, the proportion of patients who experienced acute exacerbation was higher in patients with airflow limitation than in those without (89.1 vs. 67.7%; P=0.015). The proportion of patients who experienced acute exacerbation was not associated with the extent of lung destruction (89.7% vs. 72.3% vs. 90.9%; P=0.868).
After adjusting for age, gender, BMI, smoking history, and extent of destruction, the rate of acute exacerbation (number of events/person-year) was higher in patients with airflow limitation than in those without (incidence rate ratio [IRR], 1.46; 95% CI, 1.37-1.55). When FEV1 was adjusted for, the incidence rate of acute exacerbation remained higher in patients with airflow limitation (IRR, 1.19; 95% CI, 1.11-1.27; Table 5). Among patients with acute exacerbation, 8/114 (7.0%) of those with airflow limitation were frequent exacerbators. By contrast, no frequent exacerbator existed among patients without airflow limitation; however, this was not statistically significant.
Predictive factors for acute exacerbation were evaluated by Cox regression analysis. After adjusting for age, gender, and extent of destruction, the hazard ratio (HR) for acute exacerbation in patients with airflow limitation was 1.634. The HR of BMI (X vs. X + 1) was 0.994 (Table 6).
The decline of FEV1 was lower in the group with airflow limitation than in the group without (-2 vs. -36 mL/yr; P<0.001). No significant difference in FVC decline was noted between the two groups (+0.7 vs. -19 mL/yr; P=0.210) (Fig. 2). After adjusting for initial FEV1, the difference in FVC remained non-significant (+0.6 vs. -19 mL/yr; P=0.198).
Our data suggest that airflow limitation is a risk factor for acute exacerbation in destroyed lung by TB. One of the most striking physiological sequelae of destroyed lung by TB is airflow limitation. However, the prevalence of airflow limitation in these patients has been reported to vary from 28 to 86.4% (3, 4). In the present study, the prevalence of airflow limitation in enrolled patients was 80.5%. With the addition of previously excluded patients, the prevalence of airflow limitation was 76.9%. The variable prevalence of airflow limitation could be a result of different definitions of destroyed lung by TB. Although we enrolled patients with parenchymal destruction in greater than 25% of one hemithorax, other studies did not define the extent of destruction (3) or determined a different reference value of destruction (4).
Although airflow limitation was reported to be present in as high as 86.4% of patients with destroyed lung by TB, its pathogenesis is not fully understood. The best-known cause of airflow limitation is smoking (7); therefore, this could also be a factor in airflow limitation in destroyed lung by TB. However, more than half of patients with airflow limitation in the present study were never smokers, and there was no difference in the proportion of ever-smokers between two groups. Thus, the development of airflow limitation in patients with destroyed lung by TB could not be explained solely by smoking. This finding is supported by reports that airflow limitation developed after pulmonary TB independent of smoking status (11, 12, 13). A cross-sectional study documented that airway obstruction was associated with TB in never smokers (12). Furthermore, the PREPOCOL study established a strong association between a history of TB and airflow obstruction that was higher than that with smoking (13). At the molecular level, destroyed lung by TB and COPD possess a common pathway of parenchymal destruction by matrix metalloproteinases (14).
In the current study, the incidence of acute exacerbation was higher in patients with airflow limitation than in those without, suggesting that airflow limitation is a risk factor for acute exacerbation in destroyed lung by TB. This result was supported by Cox regression analysis. As the frequency of acute exacerbation is known to correlate with FEV1 in COPD patients (15, 16, 17), we evaluated the incidence of acute exacerbation after adjusting for FEV1. The incidence of acute exacerbation remained greater in patients with airflow limitation. By contrast, the extent of parenchymal destruction was not associated with the incidence of acute exacerbation. These findings do not preclude the association of lung destruction and acute exacerbations. As a whole, 85% (135/159) of patients experienced acute exacerbation. Moreover, patients with grade 3 of parenchymal destruction were more likely to experience acute exacerbations than those with grade 2 destruction (90.9% vs. 72.3%). These results suggest that parenchymal destruction can affect acute exacerbations, although it may not be directly proportional to the degree of destruction. Therefore, it seems that the impact of airflow limitation is more important than lung destruction regarding the issue of acute exacerbations.
In the current study, another risk factor for acute exacerbation in destroyed lung by TB was BMI. This is similar to a report that risk factors for acute exacerbation in COPD included low BMI. Previous study reported that low BMI was significantly associated with failure of noninvasive ventilation and the need to intubate in acute exacerbation of pulmonary TB sequelae (18). Additionally, in COPD, the BODE index (which combines the following four variables into a composite score: [B] BMI; [O] airflow obstruction; [D] dyspnea; and [E] exercise capacity) was a predictor of mortality regarding the risk of death from both any cause and from respiratory causes (19). Additionally, a BMI <21 kg/m2 was frequent in patients hospitalized due to acute exacerbation of COPD (20).
Airflow limitation in COPD is characterized by a progressive nature. Some previous studies have reported that greater decline in absolute FEV1 occurred in the early stage of COPD. Tantucci and Modina (21) reviewed recent clinical trials to assess the decline of lung function in COPD patients with each stage according to the severity of airflow obstruction. They reported that COPD patients in the early stages had more lung function to lose than those in the most-advanced stage. The mean rates of FEV1 decline in Global Initiative for Obstructive Lung Disease (GOLD) stages II and III 47-79 mL/yr and 56-59 mL/yr, respectively, and <35 mL/yr in GOLD stage IV. In the present study, the predicted value of FEV1 in patients with airflow limitation was 45.0%±16.7%, which is comparable to COPD of GOLD stage III. The rate of decline of FEV1 in patients with airflow limitation (-2 mL/yr) is likely to be lower than that reported in COPD patients of GOLD stage III; this could be explained by smoking status. In the ECLIPSE study, 36% of enrolled patients were current smokers, and the rate of FEV1 decline was greater in current smokers than in former smokers (22). In the present study, current smokers reported only 8% (9/118) of patients. The lower number of current smokers could be due to lower rate of FEV1 decline compared with that in COPD patients. The different rate of FEV1 decline may reflect differences in the nature of airflow limitation between destroyed lung by TB and COPD.
The decline of FEV1 was lower in destroyed lung by TB with airflow limitation than that without limitation in the present study. The decline of FEV1 is proportional to FEV1 in COPD. Namely, the larger the FEV1, the faster the rate of FEV1 decline. Because FEV1 was significantly lower in patients with airflow limitation, airflow limitation could be the reason for the lower rate of FEV1 decline. However, when FEV1 was adjusted for, the rate of FEV1 decline remained lower in patients with airflow limitation, implying that the underlying cause remains unclear. Two pharmacological studies, TORCH (23) and UPLIFT (24) trials demonstrated that medical treatment including combinations of inhaled corticosteroids and long-acting beta-agonists or anticholinergic bronchodilator improved lung function in COPD patients. Although the effect of medical treatment is not known in patients with destroyed lung by TB, it is very likely that patients with airflow limitation in destroyed lung by TB might have received more aggressive treatment with bronchodilators or anti-inflammatory drugs than those without. If these drugs can reduce the rate of FEV1 decline in patients with destroyed lung by TB as in COPD patients, the lower rate of FEV1 decline in patients with airflow limitation could be explained by the difference in pharmacotherapy.
Little is known of the clinical differences in destroyed lung by TB according to airflow limitation. To our knowledge, this is the first study to compare the clinical features, particularly acute exacerbation and FEV1 decline, of patients with destroyed lung by TB with and without airflow limitation. Our study possessed some limitations. First, it was of a retrospective design. We could not evaluate patients with mixed changes separately because the initial total lung capacity data were not available. Second, the coexistence of COPD cannot be excluded because we included patients with a smoking history. However, excluding ever smokers with airflow limitation, acute exacerbation also occurred more in patients with airflow limitation. Third, pharmacotherapy was not evaluated. In COPD patients, a large body of clinical evidence has shown that long-acting bronchodilators are effective in preventing exacerbations (25, 26) and decreasing the decline of FEV1 (27, 28). Therefore, the efficacy of pharmacotherapy in destroyed lung by TB with airflow limitation should be evaluated. A well-designed prospective study without these limitations should be conducted to confirm the results of the present study.
In conclusion, the presence of airflow limitation is an independent risk factor for acute exacerbation in patients with the destroyed lung by TB. Lung function decline is more severe in the group without airflow limitation in the destroyed lung by TB.
Figures and Tables
Fig. 2
Changes of pulmonary finction tests. (A) Annual decline of FEV1. The annual decline of FEV1 was lower in patients with airflow limitation than in those without (-2 vs. -36 mL/yr, respectively; P < 0.001). (B) Annual decline of FVC. There was no significance difference in the rate of FVC decline between the two groups (+0.7 vs. -19 mL/yr; P = 0.201).

Table 1
Demographic characteristics of patients with destroyed lung by tuberculosis

Table 2
Lung function and extent of parenchymal destructions

Table 3
Demographic characteristics in patients with or without airflow limitation
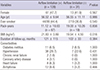
Table 4
Lung function and extent of parenchymal destruction in patients with or without airflow limitation
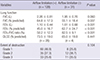
Table 5
Frequency of acute exacerbation in patients with or without airflow limitation

Data are shown as No. (%) or median [range]. *Poisson regression, IRR, 1.19; 95% CI, 1.11-1.27, adjusted by age, gender, BMI, smoking history, extent of destruction, and initial FEV1. IRR, incidence rate ratio; CI, confidence interval; BMI, body mass index; FEV1, forced expiratory volume in 1 second.
Table 6
Predicting factors for acute exacerbation

References
1. Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003; 163:1009–1021.
2. Pasipanodya JG, Miller TL, Vecino M, Munguia G, Garmon R, Bae S, Drewyer G, Weis SE. Pulmonary impairment after tuberculosis. Chest. 2007; 131:1817–1824.
3. Plit ML, Anderson R, Van Rensburg CE, Page-Shipp L, Blott JA, Fresen JL, Feldman C. Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J. 1998; 12:351–356.
4. Chae JN, Jung CY, Shim SW, Rho BH, Jeon YJ. CT Radiologic findings in patients with tuberculous destroyed lung and correlation with lung function. Tuberc Respir Dis. 2011; 71:202–209.
5. Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax. 2000; 55:32–38.
6. Lee SW, Kim YS, Kim DS, Oh YM, Lee SD. The risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosis. J Korean Med Sci. 2011; 26:268–273.
7. Chakrabarti B, Calverley PM, Davies PD. Tuberculosis and its incidence, special nature, and relationship with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2:263–272.
8. Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology. 2010; 15:623–628.
9. Lee JH, Chang JH. Lung function in patients with chronic airflow obstruction due to tuberculous destroyed lung. Respir Med. 2003; 97:1237–1242.
10. Seo YK, Lee CH, Lee HK, Lee YM, Park HK, Choi SB, Kim HG, Jang HJ, Yum HK, Lee SH. Differences between patients with TB-destroyed lung and patients with COPD admitted to the ICU. Tuberc Respir Dis. 2011; 70:323–329.
11. Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, Lam TH, Adab P. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010; 137:593–600.
12. Perez-Padilla R, Fernandez R, Lopez Varela MV, Montes de, Muiño A, Tálamo C, Brito Jardim JR, Valdivia G, Baptista Menezes AM. Airflow obstruction in never smokers in five Latin American cities: the PLATINO study. Arch Med Res. 2012; 43:159–165.
13. Caballero A, Torres-Duque CA, Jaramillo C, Bolivar F, Sanabria F, Osorio P, Orduz C, Guevara DP, Maldonado D. Prevalence of COPD in five Colombian cities situated at low, medium, and high altitude (PREPOCOL study). Chest. 2008; 133:343–349.
14. Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006; 61:259–266.
15. Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010; 363:1128–1138.
16. Cote CG, Dordelly LJ, Celli BR. Impact of COPD exacerbations on patient-centered outcomes. Chest. 2007; 131:696–704.
17. Hoogendoorn M, Feenstra TL, Hoogenveen RT, Al M, Mölken MR. Association between lung function and exacerbation frequency in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2010; 5:435–444.
18. Aso H, Kondoh Y, Taniguchi H, Kimura T, Nishiyama O, Kato K, Kataoka K, Hasegawa Y. Noninvasive ventilation in patients with acute exacerbation of pulmonary tuberculosis sequelae. Intern Med. 2010; 49:2077–2083.
19. Celli BR, Cote CG, Marin JM, Casanova C, Montes de, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004; 350:1005–1012.
20. Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, Kosnik M, Anker SD, Suskovic S. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011; 2:81–86.
21. Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012; 7:95–99.
22. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Coxson HO, Crim C, ECLIPSE Investigators, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011; 365:1184–1192.
23. Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356:775–789.
24. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M; UPLIFT Study Investigators. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359:1543–1554.
25. Wedzicha JA, Decramer M, Seemungal TA. The role of bronchodilator treatment in the prevention of exacerbations of COPD. Eur Respir J. 2012; 40:1545–1554.
26. Jochmann A, Scherr A, Jochmann DC, Miedinger D, Török SS, Chhajed PN, Tamm M, Leuppi JD. Impact of adherence to the GOLD guidelines on symptom prevalence, lung function decline and exacerbation rate in the Swiss COPD cohort. Swiss Med Wkly. 2012; 142:w13567.
27. Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009; 374:1171–1178.
28. Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Vestbo J, Knobil K, Yates JC, Calverley PM. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008; 178:332–338.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download