Abstract
Figures and Tables
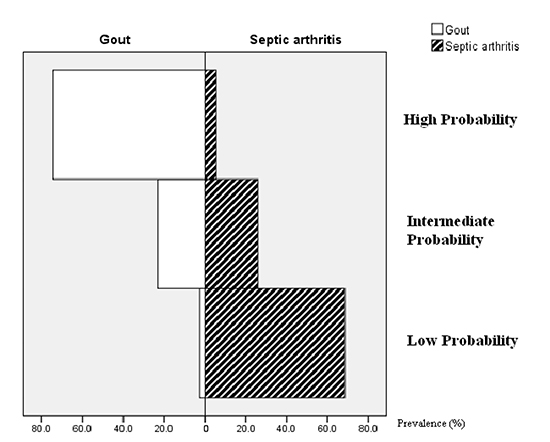
Fig. 1
Histogram of the proportion of 3 probability groups in patients with gout and septic arthritis.
Table 1
Patient characteristics
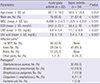
Table 2
Comparison of the positive rates of the variables of the diagnostic rule
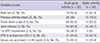
Table 3
Association of probability scores with gout diagnosis

ACKNOWLEDGMENTS
Notes
DISCLOSURE The authors have no commercial associations that might pose a conflict of interest in connection with this article.
AUTHOR CONTRIBUTION Conception and design of the work: Lee KH, Choi ST, Lee SK, Lee JH, Yoon BY. Acquisition of data: Lee KH, Lee SK. Analysis of data: Lee KH, Choi ST, Lee SK, Lee JH, Yoon BY. Drafting the work or revising the content of manuscript: Lee KH, Choi ST, Lee SK, Lee JH, Yoon BY. Wrote the paper: Lee KH. Final approval of the manuscript to be published: Lee KH, Choi ST, Lee SK, Lee JH, Yoon BY. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: Lee KH, Choi ST, Lee SK, Lee JH, Yoon BY. ICMJE criteria for authorship read and met: Lee KH, Choi ST, Lee SK, Lee JH, Yoon BY.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download