Abstract
Figures and Tables
Table 1
Univariate analyses of perinatal and neonatal factors between late-onset hyponatremia group and non-late-onset hyponatremia groups

LOH, late-onset hyponatremia; GA, gestational age; SD, standard deviation; M:F, male:female; AS, apgar score; SGA, small for gestational age; GDM, gestational diabetes mellitus; PROM, premature rupture of membrane; RDS, respiratory distress syndrome; PDA, persistent ductus arteriosus; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; Gr, grade by Volpe; BM, breast milk; TPN, total parenteral nutrition; BPD, bronchopulmonary dysplasia; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; EUGR, extrauterine growth retardation.
Table 2
Multivariate logistic regression analysis of risk factors of late-onset hyponatremia
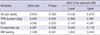
Table 3
Multivariate linear or logistic regression analyses of the influences of late-onset hyponatremia on neonatal outcomes

Table 4
Univariate analyses of perinatal and neonatal factors between the late-onset hyponatremia lasting at least 7 days group and the others

LOH, late-onset hyponatremia; GA, gestational age; SD, standard deviation; M:F, male:female; AS, apgar score; SGA, small for gestational age; GDM, gestational diabetes mellitus; PROM, premature rupture of membrane; RDS, respiratory distress syndrome; PDA, persistent ductus arteriosus; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; Gr, grade by Volpe; BM breast milk; TPN, total parenteral nutrition; BPD, bronchopulmonary dysplasia; PVL, periventricular leukomalacia; ROP, retinopathy of prematurity; EUGR, extrauterine growth retardation.
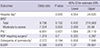
Table 5
Multivariate linear or logistic regression analyses of the influences of late-onset hyponatremia longer than 7 days on neonatal outcomes
Notes
Conception and coordination of the study: Lee JA. Design of ethical issues: Kim YJ, Lee JA. Acquisition of data: Kim YJ. Data review: Kim YJ, Lee JA. Statistical analysis: Kim YJ, Lee JA, Oh S. Manuscript preparation: Kim YJ, Lee JA, Choi CW, Kim EK, Kim HS, Kim BI, Choi JH. Manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download