1. Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF; Vermont Oxford Network ELBW Infant Follow-Up Study Group. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998-2003. Neonatology. 2010; 97:329–338.
2. Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002; 87:F37–F41.
3. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978; 92:529–534.
4. Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA, Buzas JS. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012; 129:1019–1026.
5. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010; 126:443–456.
6. McCrea HJ, Ment LR. The diagnosis, management, and postnatal prevention of intraventricular hemorrhage in the preterm neonate. Clin Perinatol. 2008; 35:777–792. vii.
7. Kaiser JR, Gauss CH, Pont MM, Williams DK. Hypercapnia during the first 3 days of life is associated with severe intraventricular hemorrhage in very low birth weight infants. J Perinatol. 2006; 26:279–285.
8. Wells JT, Ment LR. Prevention of intraventricular hemorrhage in preterm infants. Early Hum Dev. 1995; 42:209–233.
9. Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014; 41:47–67.
10. Gawlowski Z, Aladangady N, Coen PG. Hypernatraemia in preterm infants born at less than 27 weeks gestation. J Paediatr Child Health. 2006; 42:771–774.
11. Harkavy KL, Scanlon JW. Hypernatremia in the very low birthweight infant. Int J Pediatr Nephrol. 1983; 4:75–78.
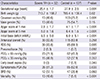
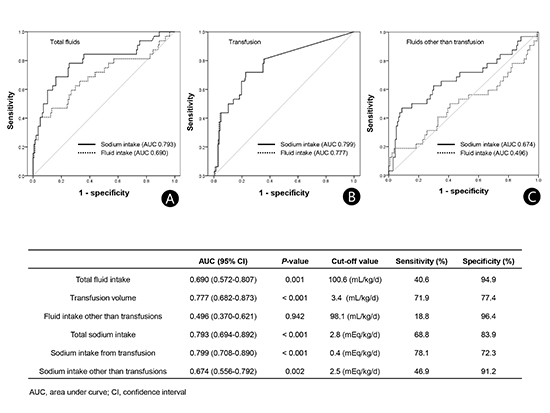
12. Barnette AR, Myers BJ, Berg CS, Inder TE. Sodium intake and intraventricular hemorrhage in the preterm infant. Ann Neurol. 2010; 67:817–823.
Hartnoll G., Bétrémieux P., Modi N. Randomised controlled trial of postnatal sodium supplementation on body composition in 25 to 30 week gestational age infants. Arch Dis Child Fetal Neonatal Ed. 2000. 82:F24–F28.
14. Lim WH, Lien R, Chiang MC, Fu RH, Lin JJ, Chu SM, Hsu JF, Yang PH. Hypernatremia and grade III/IV intraventricular hemorrhage among extremely low birth weight infants. J Perinatol. 2011; 31:193–198.
15. Costarino AT Jr, Gruskay JA, Corcoran L, Polin RA, Baumgart S. Sodium restriction versus daily maintenance replacement in very low birth weight premature neonates: a randomized, blind therapeutic trial. J Pediatr. 1992; 120:99–106.
16. Bell EF, Acarregui MJ. Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. 2008; Cd000503.
17. Simmons MA, Adcock EW 3rd, Bard H, Battaglia FC. Hypernatremia and intracranial hemorrhage in neonates. N Engl J Med. 1974; 291:6–10.
18. Wigglesworth JS, Keith IH, Girling DJ, Slade SA. Hyaline membrane disease, alkali, and intraventricular haemorrhage. Arch Dis Child. 1976; 51:755–762.
19. Roberton NR, Howat P. Hypernatraemia as a cause of intracranial haemorrhage. Arch Dis Child. 1975; 50:938–942.
20. Van de Bor M, Van Bel F, Lineman R, Ruys JH. Perinatal factors and periventricular-intraventricular hemorrhage in preterm infants. Am J Dis Child. 1986; 140:1125–1130.
21. Dykes FD, Lazzara A, Ahmann P, Blumenstein B, Schwartz J, Brann AW. Intraventricular hemorrhage: a prospective evaluation of etiopathogenesis. Pediatrics. 1980; 66:42–49.
22. Lupton BA, Roland EH, Whitfield MF, Hill A. Serum sodium concentration and intraventricular hemorrhage in premature infants. Am J Dis Child. 1990; 144:1019–1021.
23. Sabir H, Stannigel H, Mayatepek E, Hoehn T. Severe hypernatremia in an extremely low birth weight infant with subsequent normal neurological development. Neonatology. 2010; 97:90–92.
24. Filippi L, Cecchi A, Dani C, Bertini G, Pezzati M, Rubaltelli FF. Hypernatraemia induced by sodium polystyrene sulphonate (Kayexalate) in two extremely low birth weight newborns. Paediatr Anaesth. 2004; 14:271–275.
25. Perrott S, Dodds L, Vincer M. A population-based study of prognostic factors related to major disability in very preterm survivors. J Perinatol. 2003; 23:111–116.
26. Musapasaoglu H, Agildere AM, Teksam M, Tarcan A, Gurakan B. Hypernatraemic dehydration in a neonate: brain MRI findings. Br J Radiol. 2008; 81:e57–e60.
27. Han BK, Lee M, Yoon HK. Cranial ultrasound and CT findings in infants with hypernatremic dehydration. Pediatr Radiol. 1997; 27:739–742.
28. Korkmaz A, Yiğit S, Firat M, Oran O. Cranial MRI in neonatal hypernatraemic dehydration. Pediatr Radiol. 2000; 30:323–325.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download