Abstract
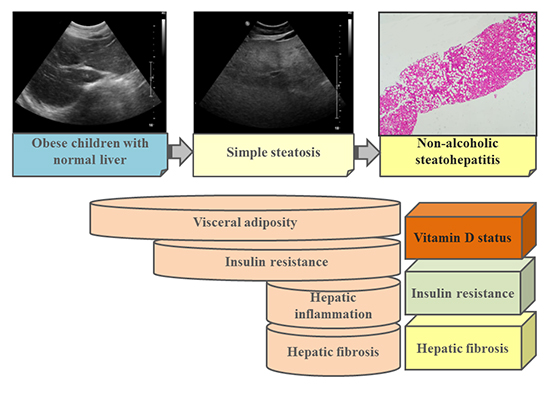
Graphical Abstract
Figures and Tables
Table 1
Clinical and biochemical profiles and vitamin D status of obese children according to the spectrum of nonalcoholic fatty liver disease

Nonparametric analysis was done using the Kruskall-Wallis method. *P value less than 0.05 is regarded as statistically significant; †The values are expressed as median (range). NASH, nonalcoholic steatohepatitis; BMI, body mass index; LDL, low density lipoprotein; HDL, high density lipoprotein; HOMA-IR, insulin resistance determined by homeostasis model assessment; HbA1c, hemoglobin A1c; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGT, γ-glutamyl transpeptidase; 25-OH Vit D, 25-hydroxy vitamin D; hsCRP, highly sensitivity C-reactive protein.
Table 2
Comparison of hepatic fibrosis scores, bone mineral density, and body fat composition among obese children according to the status of nonalcoholic fatty liver disease

Nonparametric analysis was done using the Kruskall-Wallis method. *P value less than 0.05 is regarded as statistically significant; †The values are expressed as median (range). NASH, nonalcoholic steatohepatitis; AST, aspartate aminotransferase; ALT, alanine aminotransferase; APRI, aspartate aminotransferase to platelet ratio index; FIB4, fibrosis 4; BMD, bone mineral density; TBLH, total body less head.
Table 3
Correlation of serum vitamin D levels and total age-matched bone mineral density z-score with obesity- and nonalcoholic fatty liver disease (NAFLD)-related factors in obese children with different spectrum of NAFLD

*P value less than 0.05 is regarded as statistically significant. NASH, nonalcoholic steatohepatitis; BMI, body mass index; LDL, low density lipoprotein; HDL, high density lipoprotein; HOMA-IR, insulin resistance determined by homeostasis model assessment; HbA1c, hemoglobin A1c; AST, aspartate aminotransferase; ALT, alanine aminotransferase; γGT, γ-glutamyl transpeptidase; hsCRP, highly sensitivity C-reactive protein; APRI, aspartate aminotransferase to platelet ratio index; FIB4, fibrosis 4; BMD, bone mineral density; TBLH, total body less head.
Table 4
Multiple regression analysis of serum vitamin D status and total age-matched bone mineral density in obese children with nonalcoholic steatohepatitis according to demographic, laboratory, bone mineral density, and body composition factors

| Variables | Coefficient | SE | P value* |
|---|---|---|---|
| Vitamin D | |||
| Age | -1.124 | 0.450 | 0.019 |
| HOMA-IR | -1.325 | 0.557 | 0.024 |
| Total age-matched BMD | |||
| BMI | 0.137 | 0.038 | 0.001 |
| Cholesterol | -0.010 | 0.004 | 0.030 |
ACKNOWLEDGEMENTS
Notes
AUTHOR CONTRIBUTION Conception and coordination of the study: Yang HR, Chang EJ. Design of ethical issues: Yang HR, Chang EJ. Acquisition of data: Yang HR, Chang EJ, Yi DY. Data review: Yang HR, Chang EJ, Yi DY. Statistical analysis: Yang HR, Chang EJ. Manuscript preparation: Yang HR, Chang EJ, Yi DY. Manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download