Abstract
Graphical Abstract
Figures and Tables
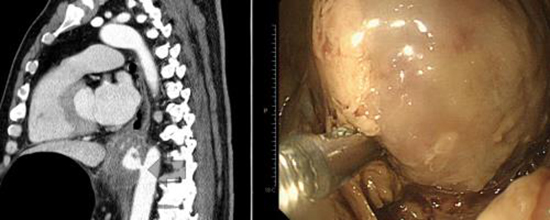
Fig. 1
A swelling was seen on the outer wall of the esophagus, through which blood leaked directly from the aorta.
Journal List > J Korean Med Sci > v.30(11) > 1022795


Hwa Kyun Shin 
https://orcid.org/http://orcid.org/0000-0003-2078-5098
Chang Woo Choi 
https://orcid.org/http://orcid.org/0000-0002-8042-7447
Jae Woong Lim 
https://orcid.org/http://orcid.org/0000-0003-4512-0236
Keun Her 
https://orcid.org/http://orcid.org/0000-0001-7824-1988