Abstract
Figures and Tables
 | Fig. 1Cumulative survival curve of the incident cases in the KORPAH (n = 297). The first-, second- and third-year estimated survival rates were 90.8%, 87.8%, and 84.4%, respectively. |
 | Fig. 2Comparison of survival according to the etiologies of PAH of the incident cases in the KORPAH (n = 297). This figure presents a comparison of prognoses according to the etiologies of PAH. PAH with CTD corresponded to the highest mortality (18.8%), followed by idiopathic PAH (IPAH) (8.1%) and PAH with congenital heart disease CHD (3.9%) (P = 0.043). CHD, congenital heart disease; CTD, connective tissue disease. |
Table 1
Baseline characteristics and comorbid conditions of KORPAH patients

P values for 6 MWD, SBP, BNP, NT proBNP, and DLCO were obtained from the independent t-test or Wilcoxon rank sum test to compare the means between functional classes I/II and III/IV. P values for categorical data were obtained from chi-square test or Fisher's exact test. NS: P>0.05. APAH, acquired pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; CHD, congenital heart disease; CTD, connective tissue disease; DM, diabetes mellitus; IHD, ischemic heart disease; COPD, chronic obstructive lung disease; TIA, transient ischemic attack; BP, blood pressure; BNP, serum brain natriuretic peptide; NT-proBNP, serum N-terminal proBNP; 6MWD, 6-minute walking distance; DLCO, diffusion capacity for carbon monoxide.
Table 2
Comparisons according to WHO functional classes in KORPAH patients
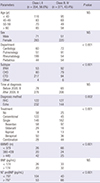
Table 3
Comparison of RHC parameters between IPAH and APAHs

NS: P>0.05. APAH, acquired pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; CHD, congenital heart disease; CTD, connective tissue disease; RHC, right heart catheterization; mPAP, mean pulmonary artery pressure; mPCWP, mean pulmonary capillary wedge pressure; mRAP, mean right arterial pressure; PVRI, pulmonary vascular resistance index; CI, cardiac index; SvO2, mixed venous oxygen saturation.
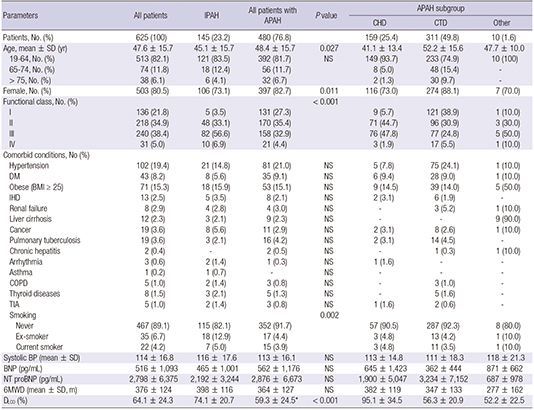
Table 4
Baseline characteristics of incident cases in KORPAH

P values for 6MWD, SBP, BNP, NT proBNP, and DLCO were obtained from the independent t-test to compare the means between patients diagnosed with IPAH and all patients with APAH. P values for categorical data were obtained from chi-square test or Fisher's exact test. NS: P>0.05. APAH, acquired pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; CHD, congenital heart disease; CTD, connective tissue disease; BP, blood pressure; BNP, serum brain natriuretic peptide; NT-proBNP, serum N-terminal proBNP; 6MWD, 6-minute walking distance; DLCO, diffusion capacity for carbon monoxide.
Table 5
Comparisons of RHC parameters between IPAH and APAH in the incident cases of KORPAH

APAH, acquired pulmonary arterial hypertension; IPAH, idiopathic pulmonary arterial hypertension; CHD, congenital heart disease; CTD, connective tissue disease; RHC, right heart catheterization; mPAP, mean pulmonary artery pressure; mPCWP, mean pulmonary capillary wedge pressure; mRAP, mean right arterial pressure; PVRI, pulmonary vascular resistance index; CI, cardiac index; SvO2, mixed venous oxygen saturation.
Table 6
PAH-specific medications of KORPAH in all patients and incident patients

Table 7
Comparisons of demographic, clinical and hemodynamic characteristics in world registries of PAH

ACKNOWLEDGMENTS
Notes
Funding This study was funded by Handok, Inc. (Seoul, Korea) and Actelion Pharmaceuticals (Allschwil, Switzerland).
DISCLOSURE The authors have no conflicts of interest to disclose. The sponsors have not involved any of the research procedures or writing.
AUTHOR CONTRIBUTION Conception and design of this study: all authors. Interpretation of results and drafting the manuscript: Chung WJ, Park YB, Jeon CH, Jung JW and Jung HO. Analysis and interpretation of data: Ko KP, Chung WJ, and Jung HO. Critical revision and final submission of manuscript: Chung WJ and Jung HO. Manuscript approval: all authors.
KORPAH INVESTIGATORS KORPAH investigators were as follows: Hae Ok Jung, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul; Wook-Jin Chung, Gachon University Gil Medical Center, Incheon; Yong Bum Park, Hallym University Medical Center, Seoul; Chan Hong Jeon, Soonchunhyang University Hospital, Bucheon; Jo-Won Jung, Severance Hospital, Yonsei University, Seoul; Kwang-Phil Ko, Gachon University College of Medicine, Incheon; Sung Jae Choi, Korea University Ansan Hospital, Ansan; Hye-Sun Seo, SoonChunHyang University Hospital, Bucheon; Jaeseung Lee, Asan Medical Center, Seoul; Sung-A Chang, Samsung Medical Center, Seoul; Sang Do Lee, Asan Medical Center, Seoul; Sang-Min Lee, Seoul National University Hospital, Seoul; Ki-Jo Kim, Yeouido St. Mary's Hospital, The Catholic University of Korea, Seoul; Kwi-Young Kang, Incheon St. Mary's Hospital, The Catholic University of Korea, Incheon; Choong-Won Lee, Wallace Memorial Baptist center, Busan; Mie-Jin Lim, Inha University Hospital, Incheon; Yong-Beom Park, Severance Hospital, Yonsei University, Seoul; Won Park, Inha University Hospital, Incheon; Sung Hwan Park, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul; Jae-Bum Jun, Hanyang University Medical Center, Seoul; Hojoong Kim, Samsung Medical Center, Seoul; Byung-Su Yoo, Wonju Severance Christian Hospital, Wonju; Young Ho Lee, Korea University Anam Hospital, Seoul; Sang-Youn Jung, Severance Hospital, Yonsei University, Seoul; Kichul Shin, Seoul National University Hospital, Seoul; Sung Ji Lee, Chonnam National University Hospital, Gwangju; Hyo-Jin Choi, Gachon University Gil Medical Center, Incheon; Hyun-A Kim, Ajou University Hospital, Suwon; Chang-Hee Suh, Ajou University Hospital, Suwon; Jong-Dae Ji, Korea University Anam Hospital, Seoul; Eun-Mi Koh, Samsung Medical Center, Seoul; Hoon-Suk Cha, Samsung Medical Center, Seoul; Kwang-Hoon Lee, Severance Hospital, Yonsei University, Seoul; Yonggil Kim, Asan Medical Center, Seoul; Shin Seok Lee, Chonnam National University Hospital, Gwangju; Seong-Su Nah, Soonchunhyang University Hospital, Cheonan; Seong-Ryul Kwon, Inha University Hospital, Incheon; Bo-Young Yoon, Inje University Ilsan Paik Hospital, Goyang; Eun-Young Lee, Seoul National University Hospital, Seoul; Hyun-Sook Kim, Chosun University Hospital, Gwangju; Ki-Won Moon, Seoul National University Hospital, Seoul; Yong Wook Park, Chonnam National University Hospital, Gwangju; Jin Ho Shin, Hanyang University Medical Center, Seoul; Joong-Kyong Ahn, Samsung Medical Center, Seoul; Dae Hyun Yoo, Hanyang University Medical Center, Seoul; Jaejoon, Lee, Samsung Medical Center, Seoul; Seong-Ho Kim, Sejong General Hospital, Bucheon; Gi-Beom Kim, Seoul National University Hospital, Seoul; I-Seok Kang, Samsung Medical Center, Seoul; Jae-Young Choi, Severance Hospital, Yonsei University, Seoul; Seong-Mi Park, Korea University Anam Hospital, Seoul; Se Joong Rim, Gangnam Severance Hospital, Yonsei University, Seoul; Sang Jea Lee, Wonkwang University School of Medicine&Hospital, Iksan; Young Hwue Kim, Asan Medical Center, Seoul; Deok-Young Choi, Gachon University Gil Medical Center, Incheon; Kye Hun Kim, Chonnam National University Hospital, Gwangju; Kyung-Im Cho, Maryknoll medical center, Busan; Se-Whan Lee, Soonchunhyang University Hospital, Cheonan; Hyoung-Doo Lee, Pusan National University Yangsan Hospital, Yangsan; Woo-Shik Kim, Kyung Hee University Medical Center, Seoul; Won Ho Kim, Chonbuk National University Hospital, Jeonju; Il-Suk Sohn, Kyung Hee University Hospital at Gandong, Seoul; Geu-Ru Hong, Yeungnam University Medical Center, Daegu; Nam Su Kim, Hanyang University Medical Center, Seoul; Yeo Hyang Kim, Keimyung University Dongsan Medical Center, Daegu; Yong-Jin Kim, Seoul National University Hospital, Seoul; Dae Gyun Park, Hallym University Medical Center, Seoul; Kwang Je Lee, Chung-Ang University, College of Medicine, Seoul; Suk-koo Choi, Inje University Seoul Paik Hospital, Seoul; Mi Young Han, Kyung Hee University Medical Center, Seoul; Myung Chul Hyun, Kyungpook National University Hospital, Daegu; Kyung-Soon Hong, Hallym University Chuncheon Sacred Heart Hospital, Chuncheon; In-Jae Oh, Chonnam National University Hospital, Gwangju; Byoung Whui Choi, Chung-Ang University, College of Medicine, Seoul; Myung-Goo Lee, Hallym University Chuncheon Sacred Heart Hospital, Chuncheon; Ji-Hyun Lee, Bundang CHA Hospital, Seongnam; and Moo Suk Park, Severance Hospital, Yonsei University, Seoul.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download