Abstract
Figures and Tables
Fig. 1
Behavioral assessments. (A) Schematic representation of behavioral assessments after intrathecal injection of nefopam. (B) Time course change of hind limb mechanical sensitivity to a dynamic plantar aesthesiometer after intrathecal injection of nefopam. (C) The change in withdrawal threshold following intrathecal injection of nefopam (10 or 100 µg/kg) every day or normal saline in rats receiving spinal nerve ligation (SNL). *P < 0.05 compared to control by one-way analysis of variance (ANOVA); †P < 0.05 compared to baseline by repeated measure ANOVA. S: sham surgery group, C: normal saline group, N10: 10 µg/kg nefopam group, N100: 100 µg/kg nefopam group, Initial: before SNL, Post-SNL: 3rd day after SNL, Post-ICI: 1st day after surgical intrathecal catheter insertion, Day-7: 7th day after intrathecal nefopam injection, Day-14: 14th day after intrathecal nefopam injection.

Fig. 2
Photographs (40 × magnification) illustrating the effect of nefopam on spinal immunoreactivity to cluster of differentiation molecule 11b (CD11b) after left L5 spinal nerve ligation. The immunohistochemical intensity of CD11b in the L5 segment decreased in a dose dependent manner following nefopam administration. (A) sham surgery group, (B) normal saline group, (C) 10 µg/kg of nefopam group, (D) 100 µg/kg of nefopam group.
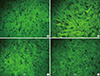
Fig. 3
Photographs (40 × magnification) illustrating the effect of nefopam on spinal immunoreactivity to glial fibrillary acidic protein (GFAP) after left L5 spinal nerve ligation. The immunohistochemical intensity of GFAP in the L5 segment decreased in a dose dependent manner following nefopam. (A) sham surgery group, (B) normal saline group, (C) 10 µg/kg nefopam group, (D) 100 µg/kg nefopam group.

Fig. 4
Changes in the relative mean optical density (MOD) ratio for (A) CD11b and (B) GFAP after left L5 spinal nerve ligation. The relative MOD ratio increased in the spinal cord side ipsilateral to the spinal nerve ligation in rats. The relative MOD ratio decreased in a dose-dependent manner in rats administered intrathecal nefopam (N10 and N100) compared to the ratio in rats administered intrathecal saline (group C). *P < 0.05 compared to the S group; †P < 0.05 compared to the C group. S: sham surgery group, C: normal saline group, N10: 10 µg/kg nefopam group, N100: 100 µg/kg nefopam group.

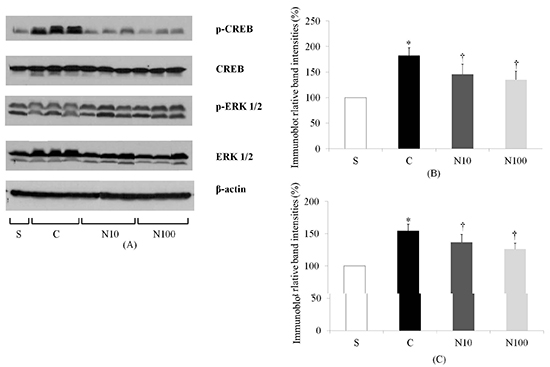
Fig. 5
The expression of extracellular signal-regulated kinase 1/2 (ERK 1/2) and cyclic adenosine monophosphate response element-binding (CREB) measured by immunoblotting. (A) Representative immunoblot images for ERK 1/2 and CREB protein in spinal cord from the S, C, N10, and N100 groups. (B, C) Densitometric quantifications of ERK 1/2 and CREB band intensities. *P < 0.05 compared to the S group; †P < 0.05 compared to the C group. S: sham surgery group, C: normal saline group, N10: 10 µg/kg nefopam group, N100: 100 µg/kg nefopam group.

Notes
This study was supported by Research institute for Convergence of biomedical science and technology (30-2014-002) Pusan National University Yangsan Hospital.
AUTHOR CONTRIBUTION Conception and design of the manuscript: Kim KH, Byeon GJ, Baek SH, Shin SW. Acquisition of data: Kim HY. Interpretation of data: Byeon GJ. Writing of the manuscript: Kim KH, Byeon GJ. Drafting the article: Kim HY, Byeon GJ, Koo ST. Final approval of the version for publishing: Shin SW. Revision of the article for important intellectual content: Koo ST.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download