Abstract
Figures and Tables
Fig. 1
Resected mediastinal mixed germ cell tumor gross specimen. The mass is well circumscribed, which corresponds to the gross feature of a teratoma. The cut surface shows necrosis, hemorrhage and cystic change.
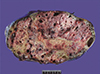
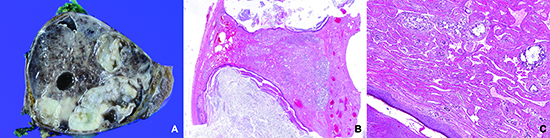
Fig. 2
Low-power view of 5 resected mediastinal germ cell tumors after chemotherapy harboring a vasculoconnective component (Hematoxylin and eosin staining, magnification, × 12.5). With variable degrees of hemorrhage, cystically delated glandular components and cartilaginous components of the mature teratoma are easily seen. Since the vasculoconnective components are intermingled with the underlying teratoma, there is no discernable mass-like lesion under low-power magnification. (A, case 1; B, case 2; C, case 3; D, case 4; E, case 5).
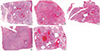
Fig. 3
Biopsy specimen before chemotherapy (A, C, E, G, I, J) and the vasculoconnective component in the resected specimen after chemotherapy (B, D, F, H, K) (Hematoxylin and eosin staining, magnification, ×40, magnification of inbox, ×200). Only two biopsy specimens (case 2, C and case 5, J) were diagnostic for a germ cell tumor (GCT). In two patients (case 2 and case 4), a variable degree of cellular atypia is found in the resected specimen (D and H, inbox). In case 5, the first biopsy shows no identifiable GCT component (I). However, in the second biopsy, an embryonal carcinoma-like area is detected (J). Histological features of the vasculoconnective components were insufficient for diagnosis of angiosarcoma (Case 1, A and B; Case 2, C and D; Case 3, E and F; Case 4, G and H; Case 5, I, J, and K).
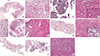
Fig. 4
A resected mediastinal mass without prior chemotherapy from an 18-yr-old male patient. Grossly, the mass is well circumscribed, and hemorrhage and cystic changes are found on the cut surface (A). Microscopically, the mass is composed of a mature teratoma, yolk sac tumor, and an abundant vasculoconnective component (B, Hematoxylin and eosin staining, magnification, ×12.5). On high-power view, the vasculoconnective component is seen to be intermingled with the yolk sac tumor (C, Hematoxylin and eosin staining, magnification, ×100). An epidermal element with keratin is seen in the left lower corner (C).

Table 1
Initial distribution and histologic diagnosis according to specimen type and gender
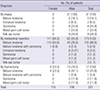
Table 2
Comparison of pathological diagnoses before and after chemotherapy in 14 patients that underwent surgical resection

*Patients who were diagnosed with a mixed GCT based on clinical and pathological information including serum tumor markers and histology; †Among these 3 patients, one received two biopsies because the initial biopsy revealed only fibrovascular tissue. YST, yolk sac tumor; MT, mature teratoma; IMT, immature teratoma; GCT, germ cell tumor.
Table 3
Clinicopathological profile of 8 patients who underwent surgical resection for a mediastinal mass after chemotherapy

M, male; YST, yolk sac tumor; GCT, germ cell tumor; MT, mature teratoma; IMT, immature teratoma; AFP, alpha-feto protein; bHCG, beta human chorionic gonadotrophin; BEP, bleomycin, etoposide, cisplatin; NA, not assessed; NED, no evidence of disease; CTx, chemotherapy; FU, follow-up period; mo, months.
Notes
AUTHOR CONTRIBUTION Conception and coordination of the study: Han J. Design of ethical issues: Cha YJ, Han J. Acquisition of clinicopathological and experimental data: Cha YJ, Lee KS, Shim YM, Han J. Analysis and interpretation of data: Cha YJ, Han J. Manuscript preparation: Cha YJ, Han J. Critical review of manuscript: Cha YJ, Lee KS, Shim YM, Han J. Manuscript approval: all authors.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download