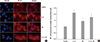
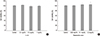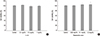1. Halim AS, Emami A, Salahshourifar I, Kannan TP. Keloid scarring: understanding the genetic basis, advances, and prospects. Arch Plast Surg. 2012; 39:184–189.
2. Jeon MK, Kang SJ, Sun H. Platysma flap with z-plasty for correction of post-thyroidectomy swallowing deformity. Arch Plast Surg. 2013; 40:425–432.
3. Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999; 7:79–89.
4. Janis JE, Harrison B. Wound healing: part I. Basic science. Plast Reconstr Surg. 2014; 133:199e–207e.
5. Sivamani RK, Garcia MS, Isseroff RR. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front Biosci. 2007; 12:2849–2868.
6. McKay IA, Leigh IM. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol. 1991; 124:513–518.
7. Ebihara N, Matsuda A, Nakamura S, Matsuda H, Murakami A. Role of the IL-6 classic- and trans-signaling pathways in corneal sterile inflammation and wound healing. Invest Ophthalmol Vis Sci. 2011; 52:8549–8557.
8. Wong VW, Gurtner GC, Longaker MT. Wound healing: a paradigm for regeneration. Mayo Clin Proc. 2013; 88:1022–1031.
9. Ghazizadeh M. Essential role of IL-6 signaling pathway in keloid pathogenesis. J Nippon Med Sch. 2007; 74:11–22.
10. Squarize CH, Castilho RM, Sriuranpong V, Pinto DS Jr, Gutkind JS. Molecular cross-talk between the NFkappaB and STAT3 signaling pathways in head and neck squamous cell carcinoma. Neoplasia. 2006; 8:733–746.
11. Brandt C, Pedersen BK. The role of exercise-induced myokines in muscle homeostasis and the defense against chronic diseases. J Biomed Biotechnol. 2010; 2010:520258.
12. Averbeck M, Gebhardt CA, Voigt S, Beilharz S, Anderegg U, Termeer CC, Sleeman JP, Simon JC. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J Invest Dermatol. 2007; 127:687–697.
13. Nyman E, Huss F, Nyman T, Junker J, Kratz G. Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid: in vitro studies of re-epithelialisation in human skin wounds. J Plast Surg Hand Surg. 2013; 47:89–92.
14. Olczyk P, Komosińska-Vassev K, Winsz-Szczotka K, Kuźnik-Trocha K, Olczyk K. Hyaluronan: structure, metabolism, functions, and role in wound healing. Postepy Hig Med Dosw (Online). 2008; 62:651–659.
15. Ewins BA, Vassiliadou M, Minihane AM, Rimbach GH, Weinberg PD. Techniques for quantifying effects of dietary antioxidants on transcription factor translocation and nitric oxide production in cultured cells. Genes Nutr. 2006; 1:125–131.
16. Price RD, Berry MG, Navsaria HA. Hyaluronic acid: the scientific and clinical evidence. J Plast Reconstr Aesthet Surg. 2007; 60:1110–1119.
17. Yang JD, Choi DS, Cho YK, Kim TK, Lee JW, Choi KY, Chung HY, Cho BC, Byun JS. Effect of amniotic fluid stem cells and amniotic fluid cells on the wound healing process in a white rat model. Arch Plast Surg. 2013; 40:496–504.
18. Tolg C, Hamilton SR, Nakrieko KA, Kooshesh F, Walton P, McCarthy JB, Bissell MJ, Turley EA. Rhamm-/- fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol. 2006; 175:1017–1028.
19. Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J Biol Chem. 2002; 277:4589–4592.
20. Teranishi S, Kimura K, Nishida T. Role of formation of an ERK-FAK-paxillin complex in migration of human corneal epithelial cells during wound closure in vitro. Invest Ophthalmol Vis Sci. 2009; 50:5646–5652.
21. Sung HJ, Kim Y, Kang H, Sull JW, Kim YS, Jang SW, Ko J. Inhibitory effect of Trolox on the migration and invasion of human lung and cervical cancer cells. Int J Mol Med. 2012; 29:245–251.
22. Jiang GX, Zhong XY, Cui YF, Liu W, Tai S, Wang ZD, Shi YG, Zhao SY, Li CL. IL-6/STAT3/TFF3 signaling regulates human biliary epithelial cell migration and wound healing in vitro. Mol Biol Rep. 2010; 37:3813–3818.
23. Prosdocimi M, Bevilacqua C. Exogenous hyaluronic acid and wound healing: an updated vision. Panminerva Med. 2012; 54:129–135.
24. Rost MS, Sumanas S. Hyaluronic acid receptor Stabilin-2 regulates Erk phosphorylation and arterial--venous differentiation in zebrafish. PLoS One. 2014; 9:e88614.
25. Hwang YP, Yun HJ, Choi JH, Han EH, Kim HG, Song GY, Kwon KI, Jeong TC, Jeong HG. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol Nutr Food Res. 2011; 55:594–605.
26. Sun H, Calle E, Chen X, Mathur A, Zhu Y, Mendez J, Zhao L, Niklason L, Peng X, Peng H, et al. Fibroblast engraftment in the decellularized mouse lung occurs via a β1-integrin-dependent, FAK-dependent pathway that is mediated by ERK and opposed by AKT. Am J Physiol Lung Cell Mol Physiol. 2014; 306:L463–L475.
27. Park D, Kim Y, Kim H, Kim K, Lee YS, Choe J, Hahn JH, Lee H, Jeon J, Choi C, et al. Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFbeta receptor interaction via CD44-PKCdelta. Mol Cells. 2012; 33:563–574.
28. Hatano H, Shigeishi H, Kudo Y, Higashikawa K, Tobiume K, Takata T, Kamata N. RHAMM/ERK interaction induces proliferative activities of cementifying fibroma cells through a mechanism based on the CD44-EGFR. Lab Invest. 2011; 91:379–391.
29. Wang C, Thor AD, Moore DH 2nd, Zhao Y, Kerschmann R, Stern R, Watson PH, Turley EA. The overexpression of RHAMM, a hyaluronan-binding protein that regulates ras signaling, correlates with overexpression of mitogen-activated protein kinase and is a significant parameter in breast cancer progression. Clin Cancer Res. 1998; 4:567–576.
30. Tian B, Brasier AR. Identification of a nuclear factor kappa B-dependent gene network. Recent Prog Horm Res. 2003; 58:95–130.
31. Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007; 8:49–62.
32. Miyazaki T, Katagiri H, Kanegae Y, Takayanagi H, Sawada Y, Yamamoto A, Pando MP, Asano T, Verma IM, Oda H, et al. Reciprocal role of ERK and NF-kappaB pathways in survival and activation of osteoclasts. J Cell Biol. 2000; 148:333–342.