INTRODUCTION
MATERIALS AND METHODS
Study devices
Animal study
Animal preparation
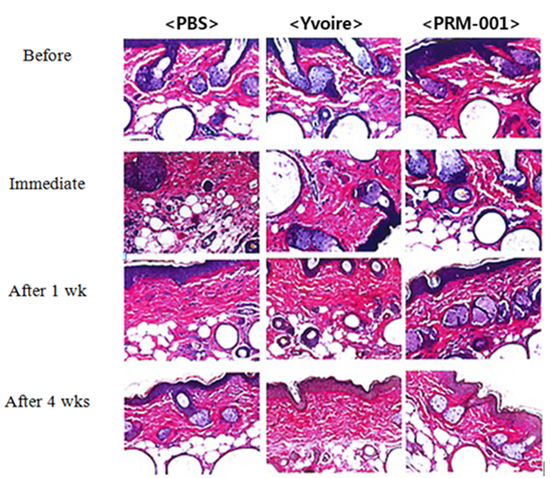
Durability
Efficacy
Clinical trial
Patient selection
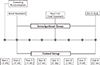
Study flowchart
Treatment procedure
Efficacy assessment
Primary objective
Secondary objectives
Changes in improvement at two and four weeks after the final treatment compared with screening, according to the judgment of investigators.
Changes in wrinkle severity (lateral canthal line severity scale) scores at two and four weeks after the final treatment compared with screening, according to the judgment of investigators
Changes in resting state wrinkle severity (lateral canthal line severity scale) scores at two and four weeks after the final treatment compared with screening, according to the judgment of investigators
Changes in visual analogue scores at two, four, and twelve weeks after the final treatment compared with screening, according to the judgment of the study subjects.




 PDF
PDF Citation
Citation Print
Print








 XML Download
XML Download