Abstract
Figures and Tables
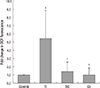 | Fig. 1Effects of CoCl2 pre-treatment on TNF-α/IFN-γ-induced ROS generation. TNF-α/IFN-γ-induced increases in intracellular ROS were revealed by the fluorescence intensity of 2',7'-dichlorofluorescein. The fluorescence intensities of the cells are shown as the relative intensities of the experimental groups compared with control cells. A representative set of three independent experiments is shown. The results are expressed as the mean±s.d. *P<0.05, compared with control cells. †P<0.05, compared with TNF-α-+INF-γ-treated cells. C, control cells; TI, TNF-α/IFN-γ-treated cells; TIC, TNF-α/IFN-γ-treated cells with CoCl2 pre-treatment; Co, CoCl2-pre-treated cells. |
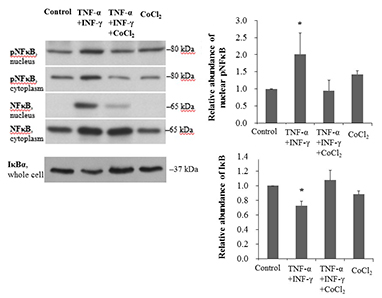
 | Fig. 2Effects of CoCl2 pre-treatment on TNF-α/IFN-γ-induced NF-κB activation. (A) Western blotting of NF-κB, phosphorylated NF-κB, and IκBα in nuclear and cytoplasmic extracts of HK-2 cell lysates. The intensities of the bands corresponding to phosphorylated NF-κB in nuclear extracts and IκBα in whole cell lysates are shown as the relative intensities of experimental groups compared with control cells. (B) The DNA-binding activity of NF-κBp50 was analyzed in the nuclear extracts of HK-2 cells using ELISA. (C) NF-κB-driven transcription is expressed as relative luciferase activity in HK-2 cells. A representative set of three independent experiments is shown. The results are expressed as the mean±s.d. *P<0.05, compared with control cells. †P<0.05, compared with TNF-α-+IFN-γ-treated cells. C, control cells; TI, TNF-α/INF-γ-treated cells; TIC, TNF-α/IFN-γ-treated cells with CoCl2 pre-treatment; Co, CoCl2-pre-treated cells. |
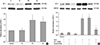 | Fig. 3The expression of hemeoxygenase-1 (HO-1). (A) Western blotting of HO-1 in HK-2 cells. HK-2 cells were pre-treated with 150 µM CoCl2 for 24 hr and then treated with 5 ng/mL TNF-α and 50 ng/mL IFN-γ for an additional 24 hr. Beta-actin was used as a loading control. A representative set of three independent experiments is shown. The densitometric ratios of HO-1 and β-actin in cells are shown as the relative ratios of experimental groups compared with control cells. The results are expressed as the mean ± s.d. *P<0.01, compared with control cells. C, control cells; TI, TNF-α/IFN-γ-treated cells; TIC, TNF-α/IFN-γ-treated cells with CoCl2 pre-treatment; Co, CoCl2-pre-treated cells. (B) Western blotting of HO-1 in HK-2 cells. GAPDH was used as a loading control. A representative set of three independent experiments is shown. The densitometric ratios of HO-1 and GAPDH in cells are shown as the relative ratios of experimental groups compared with control cells. The results are expressed as the mean±s.d. *P<0.001, compared with control cells. †P<0.005, compared with CoCl2-pre-treated cells. |
 | Fig. 4Effects of CoCl2, an NF-κB inhibitor, and HO-1 RNA interference on TNF-α/IFN-γ-induced cytokine production. (A) The production of RANTES and MCP-1 was analyzed in the supernatants of HK-2 cells using ELISA. A representative set of three independent experiments is shown. The results are expressed as the mean ± s.d. *P<0.05, compared with control cells. †P<0.05, compared with TNF-α-+IFN-γ-treated cells. C, control cells; TI, TNF-α/IFN-γ-treated cells; TIC, TNF-α/IFN-γ-treated cells with CoCl2 pre-treatment; TICN, TNF-α/IFN-γ-treated cells with CoCl2 pre-treatment and negative control siRNA transfection; TIC-HO1, TNF-α/IFN-γ-treated cells with CoCl2 pre-treatment and HO-1 siRNA transfection; Co, CoCl2-pre-treated cells. (B) HK-2 cells were pre-treated with different doses of the NF-κB inhibitor PDTC for 2 hr before exposure to TNF-α and IFN-γ. TI, TNF-α/IFN-γ-treated cells; PDTC, pyrrolidinedithiocarbamate. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download