Abstract
Figures and Tables
Fig. 1

Fig. 2
Table 1

Completeness: completeness of data among participants, E24UNA_T: estimated value of 24-hr urine sodium amount with the equation proposed by Tanaka et al., E24UNA_K: estimated value of 24-hr urine sodium amount with the equation proposed by this study. Mean-2SD: value of mean of E24UNA subtracted by 2×the standard deviation of E24UNA. Mean+2SD: value of mean of E24UNA plus 2×the standard deviation of E24UNA. Diagnosed year: time difference between current age and the age at diagnosis (yr). Present illness: participants with an illness at the time of the survey. Recent cancer: participants with cancer diagnosed within 5 yr.
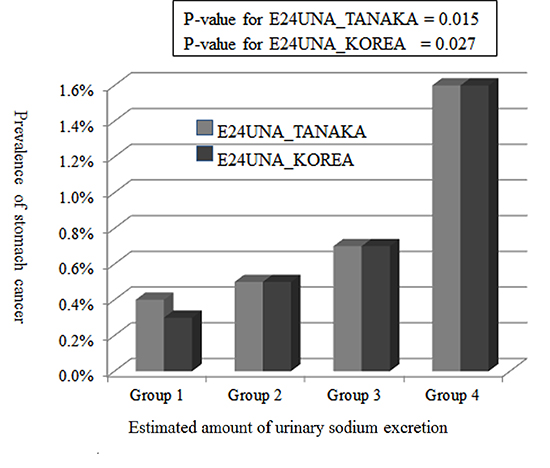
Table 2

E24UNA_T: estimated 24-hr urine sodium with the equation proposed by Tanaka et al. E24UNA_K: estimated 24-hr urine sodium with the equation proposed in this study. Group 1: E24UNA of ≤71.0 mEq/day for TANAKA; E24UNA of ≤95.0 mEq/day for KOREA. Group 2: E24UNA, 71.01-144.0 mEq/day for TANAKA; E24UNA, 95.1-167.0 mEq/day for KOREA. Group 3: E24UNA, 144.1-261.0 mEq/day for TANAKA; E24UNA, 167.1-240.0 mEq/day for KOREA. Group 4: E24UNA of ≥261.1 mEq/day for TANAKA; E24UNA of ≥240.1 mEq/day for KOREA. All: all of participants with stomach cancer history. Present: participants with current stomach cancer. Recent: participants with stomach cancer diagnosed within 5 yr. P value for All stomach cancer by E24UNA_T=0.015. P value for All stomach cancer by E24UNA_K=0.027. P value for All stomach cancer between Group 1-3 and Group 4 by E24UNA_T=0.013. P value for All stomach cancer between Group 1-3 and Group 4 by E24UNA_K=0.019.
Table 3

95% CI for OR: 95% confidence interval for the odds ratio. E24UNA_K: a continuous variable of the estimated 24-hr urine sodium excretion in 100 mEq/day unit, calculated with the equation created in this study. E24UNA_K2: a group of E24UNA_K stratified with a criterion of 240 mEq/day. E24UNA_K2 (high): participants with ≥241 mEq/day of E24UNA_K. E24UNA_T: a continuous variable of estimated 24-hr urine sodium in 100 mEq/day unit, calculated with the equation created by Tanaka et al. E24UNA_T2 (high): participants with ≥261 mEq/day, as calculated by using the E24UNA_T model. Model 1: logistic regression for the presence of stomach cancer adjusted for age and sex. Model 2: adjusted for age, sex, and smoking and drinking habits. Model 3: adjusted for age, sex, smoking and drinking habits, grade of household income, marital status, employment status, grade of education-year, history of ischemic heart disease, colon cancer, lung cancer, presence of diabetes mellitus, hypertension, and chronic kidney disease at the time of the survey.
Table 4
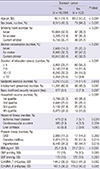
E24UNA_T: a continuous variable of estimated 24-hr urine sodium, calculated with the equation created by Tanaka et al., E24UNA_K: a continuous variable of estimated 24-hr urine sodium, calculated with the equation created in this study, Household income: Quartile group of household income defined by the Korean government according to number of household members. BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure; CKD, chronic kidney disease.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download