Abstract
The gastrointestinal tract is the most common primary extranodal site for diffuse large B-cell lymphoma (DLBCL). However, there is no consensus on the most appropriate staging system for intestinal DLBCL. We evaluated the utility of the modified Ann Arbor system, the Lugano system, and the Paris staging system (a modification of the Tumor, Node, Metastases [TNM] staging for epithelial tumors) in 66 cases of resected intestinal DLBCL. The cases were treated with surgery, plus either cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) chemotherapy alone (n=26) or with the addition of rituximab immunotherapy (n=40). Median follow-up time was 40.4 months (range, 2.1-171.6 months). Fifty-six patients (84.8%) achieved complete remission. The overall 5-yr survival rate was 86.4% (57/66). Of the stage categories defined for each staging system, only the T stage of the Paris classification showed prognostic significance for overall survival by univariate analysis. However, none of the stage parameters was significantly correlated with patient survival on multivariate analysis. In conclusion, the results suggest that the T stage of the Paris classification system may be a prognostic indicator in intestinal DLBCL. The results also imply that in surgically resected intestinal DLBCL, the addition of rituximab to the CHOP regimen does not confer significant survival advantage.
The gastrointestinal tract is the most common site of extranodal involvement for diffuse large B-cell lymphoma (DLBCL) (1). However, because of the lack of large-scale prospective randomized studies, there is no consensus on the optimal treatment against primary gastrointestinal DLBCL (2-4). Currently, the procedure of choice for intestinal DLBCL is widely considered to be a combination of surgery followed by cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) or rituximab plus CHOP (R-CHOP) chemotherapy, primarily because the preoperative diagnosis is difficult and risk of complications requiring surgery is relatively high during chemotherapy (2, 3, 5-9). The role of surgery in intestinal DLBCL has been examined prospectively, but has not been confirmed in a randomized study (7, 8). A recent large-scale retrospective study reported that in patients with localized intestinal DLBCL, surgery plus chemotherapy was associated with a lower rate of relapse than chemotherapy alone, with similar survival duration in patients treated with CHOP and R-CHOP (9). However, there have so far been no clinicopathologic studies that also take the histopathologic status of the excised specimen into consideration.
There is also a lack of consensus regarding the best staging system for gastrointestinal DLBCL. Gastrointestinal DLBCL has a different dissemination pattern from its nodal counterparts, which limits the use of the conventional Ann Arbor staging system, and various modifications have been proposed to aid the staging of gastrointestinal lymphomas, including those of Musshoff, and of the Lugano Workshop (10-12). On the other hand, the Paris staging system formulated by the European Gastro-Intestinal Lymphoma Study (EGILS) Group, is a modification of the Tumor, Node, Metastases (TNM) staging system for epithelial tumors (5, 13). Reaching a consensus has been further confounded by different authors using different definitions of localized disease and, furthermore, the staging systems have rarely been validated for R-CHOP treatment. Table 1 summarizes the various staging systems.
The aim of this study was to evaluate the prognostic utility of the Musshoff modified Ann Arbor staging system, Lugano staging systems, and the Paris TNMB staging system, in intestinal DLBCL with resection. We also investigated the survival advantage of rituximab administration for patients with intestinal DLBCL treated with a combination of surgery and CHOP or a CHOP-like regimen.
From January 1996 to March 2011, a total of 106 cases of intestinal DLBCL, not otherwise specified (NOS) satisfied the definition of primary gastrointestinal lymphoma of Lewin et al. (14). These included 66 cases treated with surgery plus post-operative chemotherapy using CHOP or R-CHOP regimens, which formed the study group. According to the anatomic distribution definitions of Koch et al. (15), 15 cases had disease of the small intestine and 51 had ileocecal disease. There was no case of colonic or rectal primary DLBCL. Staging work-up included esophagogastroduodenoscopy, colonoscopy, pharyngeal examination, computed tomography (CT) scans of chest, abdomen, and pelvis, and bone marrow examination. Positron emission tomography-computed tomography (PET-CT) was also performed for staging in 44 of the 66 patients (66.7%). Initial surgical resection was followed by three to eight cycles of post-operative chemotherapy (median, six cycles) in all of the patients except one.
Medical records were reviewed for clinical characteristics, including age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, B symptoms, serum lactate dehydrogenase (LDH) levels, bulky disease, extent of surgical resection (complete or incomplete removal of the tumor), and involvement of other extranodal sites. As gastrointestinal involvement may have resulted in poor oral intake, weight loss per se was not regarded as a B symptom. Bulky disease was defined as a mass with a diameter greater than 10 cm. Based on these factors, the International Prognostic Index (IPI) was calculated as originally described for nodal lymphomas using Musshoff modified Ann Arbor staging.
Representative sections were reviewed by two pathologists for diagnosis of DLBCL, NOS according to the 2008 World Health Organization (WHO) classification (1). The aggressive variants of large B-cell lymphoma, such as plasmablastic lymphoma or ALK-positive large B-cell lymphoma, were excluded by immunohistochemistry. Burkitt lymphoma with characteristic medium-sized B-cells with monomorphic nuclei, uniform nuclear positivity on Ki-67 immunostaining, and expression of CD10 and BCL6 without BCL2 or MUM1 expression were excluded from the study (1, 16, 17). In cases where CD10, BCL6, and MUM1 expression data were available, the cases were classified according to the cell of origin into germinal center B-like subtype (GCB) or non-GCB subtype using the algorithm of Hans et al. (18). The depth of tumor infiltration and extent of nodal involvement were documented as parameters for stage classification in the three staging systems (Fig. 1). For the Paris system which is based on the 6th edition of the TNM system by AJCC (19), we assigned the tumor stage as follows; T1 for tumor confined to mucosa (M) and/or submucosa (SM), T2 for tumor that infiltrates muscularis propria (PM) and/or subserosa (SS), T3 for tumor that infiltrates serosa (SI), and T4 for tumor that perforates serosa, or invades adjacent organs. In addition, we also assigned the tumor stage as described in the 7th edition of the TNM system; T1, SM; T2, PM; T3, SS; T4a, serosa infiltration/perforation; T4b, invasion of adjacent organs (modified Paris T stage) (20). Combined with the imaging results, the stage of individual cases was reassessed according to the modified Ann Arbor, Lugano, and Paris TNMB staging systems.
Response to treatment was defined according to the WHO criteria (21). Complete remission (CR) was defined as complete disappearance of clinical evidence of disease and absence of any new tumor lesions. Partial remission (PR) was defined as a decrease in size of at least 50% in each lesion. Progressive disease (PD) or relapse was defined as a newly developed lesion or an increase of more than 25% in the product of two diameters of at least one tumor. Progression-free survival (PFS) was defined as the time from the date of initial diagnosis to the date of progression, recurrence, death, or last follow-up. Overall survival (OS) was defined as the time from the date of initial diagnosis to the date of death or last follow-up.
The chi square test was used to evaluate the relationships between clinical features and outcomes. Survival was estimated using the Kaplan-Meier method, and differences in survival curves were evaluated by the Breslow test. Statistical evaluations were performed using SPSS statistics 17.0 software (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as a two-sided P value of less than 0.05.
The characteristics of the patients are summarized in Table 2. The median age was 54.0 yr (range, 17-77 yr). The male to female ratio was 2.67. Surgical resection of the primary tumor was performed with or without lymph node dissection and identifiable tumor foci were completely removed in 40 cases (60.6%). Of the remaining 26 cases showing residual disease, intra-abdominal lymphoma was found in 13 cases and extra-abdominal lymphoma in the remaining 13. Emergency surgery was performed in five patients (7.6%), for visceral perforation in three patients, and for intussusceptions in two patients. B symptoms and a high serum LDH level were present in ten and 25 patients, respectively. Twenty-two patients presented with a bulky mass. Most patients had a good performance status and localized disease with a low IPI score at presentation. Twenty-five cases (23.1%) exhibited an additional focus of extranodal involvement. Histopathology revealed a low-grade B-cell lymphoma component, in a background of DLBCL in two cases (3.0%), one of follicular lymphoma and one of extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In the 51 cases with CD10, BCL6, and MUM1 immunohistochemical expression data, nine (17.6%) were GCB type and 42 (82.4%) were non-GCB, according to the algorithm of Hans et al. (18).
After surgical resection, 26 patients received CHOP (39.4%), and 40 patients received R-CHOP (60.6%). The follow-up time ranged from 2.1-171.6 months (median, 40.4 months). Fifty-six patients (84.8%) achieved CR. Despite achieving remission, four of these patients relapsed within the median disease-free period of 15 months (range, 8-33 months). In all four, the relapse occurred in a distant lymph node location (aortocaval, inguinal, or thoracic region) with or without extranodal involvement. They received salvage chemotherapy with ifosfamide or etoposide-based regimens. Of these, only one patient achieved a second CR and the other three died of disease. Three patients (4.5%) reached a PR as a result of the initial chemotherapy; one patient with a disseminated disease involving peritoneum and extra-abdominal nodes at operation which resolved after the second-line chemotherapy, and two patients with residual intra-abdominal nodes after initial chemotherapy, one of which achieved a long-term remission on second-line chemotherapy, and the other succumbed to PD. In seven paients (10.6%), disease progressed without achieving CR or PR despite chemotherapy. Three of these patients had locally advanced disease with or without adjacent lymph node involvement, and the other four patients had distant metastases involving extranodal sites (peritoneum or lung) or multiple lymph nodes. Four of these seven patients received salvage chemotherapy without additional surgery; long-term remission was induced in one patient and the other three died. Of the other three patients, additional surgery induced long-term remission in one without salvage chemotherapy, but the other two patients died during conservative care. In total, nine patients (13.6%) died of disease within the median follow-up period of 16.5 months (range, 2.1-45.0 months). The overall 5-yr survival rate was 86.4% (57/66).
On univariate analysis, IPI risk group (Fig. 2A) and ECOG performance score (Fig. 2B) were significantly associated with both OS and PFS (Table 2). The analysis also revealed that the OS and PFS for R-CHOP-treated patients were not significantly different from those of CHOP-treated patients. None of the other clinical and pathologic parameters, such as complete resection, bulky disease, or B symptoms, reached statistical significance on OS and PFS. Furthermore, there was no difference in the OS or PFS of patients with GCB-type disease compared to those with non-GCB DLBCL (OS, P=0.332; PFS, P=0.214).
The distribution of the patients according to the three different staging systems, and the distribution of the tumor depth categories are summarized in Table 3. In univariate analysis, there was no significant difference in OS between patients staged according to the modified Ann Arbor system or to the Lugano system. In addition, there was no correlation of PFS with stage when classified according to either the modified Ann Arbor or the Lugano system. Using the Paris staging system, only the T parameter exhibited a statistically significant correlation with OS by univariate analysis (P<0.05; Fig. 3A). This tendency for T parameter was also maintained in the 40 patients using rituximab (P<0.05). The M parameter showed a trend towards a significant correlation with OS, although patients staged as M1 survived longer than those staged as M0 (P=0.061; Fig. 3C). None of the parameters of the Paris system were significantly correlated with PFS, although bone marrow involvement was on the borderline (P=0.051). Analysis of the tumor depth according to the modified Paris T stage did not show any significant difference in patient survival. Interestingly, the survival curves for patients with tumors invading M, SM, and PM layers showed complete overlap, while those for patients with tumors of SS and SI type were separated (Fig. 3D).
Cox regression analysis was performed to confirm the independency of the T parameter of the Paris classification system on patient's survival, but none of the parameters exhibited significant result.
We, like others (7-9, 22), have shown that intestinal DLBCL treated with surgery plus CHOP or R-CHOP chemotherapy has an excellent prognosis; in this study most of the patients (86.4%) achieved a CR and the 5-yr survival rate was 86.4%. Most cases had localized disease and a good ECOG performance status, which reflects the elective nature of the surgery in the vast majority of cases. Most importantly, survival duration was similar in patients treated with CHOP and R-CHOP, and suggests that rituximab does not confer significant survival benefit to patients with intestinal DLBCL undergoing surgical resection, in keeping with the recent report (9). Although the conventional IPI risk group and ECOG performance score were correlated with OS on univariate analysis, multivariate analysis failed to demonstrate the independency of these parameters.
The use of different staging systems for intestinal DLBCL hampers comparisons of data in the literature (4, 23, 24). Moreover, the definitions of limited disease and of extended/advanced disease vary among the authors (15, 24). Using the Lugano system, localized disease has been defined as stage I/II1; stage I/II1, and II2; or as stage I/II1, II2, and IIE by different authors (12, 15, 25). The issue is further complicated by the E designation having a different meaning in the modified Ann Arbor and the Lugano systems (10-12). Survival curves for Lugano stages II1, II2, and IIE in our study were indistinguishable, suggesting that it is appropriate for them to be considered as one single stage. However, neither the modified Ann Arbor staging nor the Lugano staging retained prognostic significance on univariate analysis.
Our study has demonstrated the relevance of the T parameter in the Paris staging system as a promising prognostic factor in resected intestinal DLBCL. Shimodaira et al. (26) first suggested the use of a TNM-based staging system for gastric lymphoma in 1994. In 2003, the EGILS Group proposed the so-called Paris staging system based on the contemporaneous TNM staging system for epithelial tumors (6th edition), which systematically describes the depth of tumor invasion of the organ wall and adjacent tissue, as well as nodal metastasis (13). Both the adaptations of the TNM system were initially proposed to satisfy the need to stage MALT lymphoma as part of the pre-treatment endoscopic ultrasound evaluation, and post-operative pathologic examination. The present study shows that the Paris system can also be useful for staging intestinal DLBCL. Histopathologic evaluation of depth of invasion in resected intestinal DLBCL may be helpful in predicting post-chemotherapeutic outcome.
Based on a large number of reports showing significantly different metastatic potential of cancers invading the PM and SS, the T stage criteria of the 6th TNM classification for the intestinal carcinomas (T1, SM; T2a, PM; T2b, SS; T3, serosal infiltration; T4, serosa perforation, invasion of adjacent organs) were changed in the 7th TNM classification (T1, SM; T2, PM; T3, SS; T4a, serosa infiltration/perforation; T4b, invasion of adjacent organs) (19, 20). In our study, distinct survival curves for PM and SS were observed, which suggests that the T2 stage of intestinal DLBCL is not be a homogeneous prognostic group. Further pathologic studies on the correlation between depth of invasion and prognosis of intestinal DLBCL are necessary to determine the homogeneity of the Paris T2 stage.
Unlike the T stage, the N stage of the Paris classification system did not correlate with survival. In fact, pathologic criteria such as node involvement, that require a laparotomy for staging are not considered to be a useful procedure in malignant lymphoma now that imaging is reliable (20). However, imaging procedures may not always detect micrometastases in lymph nodes or may cause confusion if a borderline lesion is visualized, leading to under or over staging. Furthermore, because the Paris staging system is not widely known, surgical staging of intestinal DLBCL is likely to have been overlooked in many cases, thereby hindering the coherency of the N stage. A well-controlled study of the Paris N stage might deduce significance of the surgical/pathologic stage in intestinal DLBCL.
A major limitation of the present study is its retrospective nature with non-uniform indications for surgery and chemotherapy regimens. Due to the rarity of the disorder, this series spanned 15 yr during which time there have been significant changes in attitude towards surgery and chemotherapy regimens. Although rituximab was available in Korea in 1993, it only became the treatment of choice for both old and young patients with CD20-positive DLBCL in 2002 when National Insurance started to cover its use for DLBCL in all ages; this accounts for the concentration of R-CHOP-treated patients in the latter half of the test period. Additionally, there was a selection bias among the patients treated with R-CHOP towards predominantly older patients, with a higher ECOG performance score, and higher stages, which we ascribe to the tendency of oncologists to add rituximab to CHOP in such patients. Our results, therefore, suggest that a larger scale study would be warranted.
In conclusion, our study shows that intestinal DLBCL treated by surgery plus chemotherapy has excellent prognosis in both CHOP and R-CHOP-treated patients, suggesting that rituximab does not confer significant benefit in this patient population. Of the three staging systems applied to intestinal DLBCL with resection, only the Paris T stage showed prognostic significance by univariate analysis, but not by multivariate analysis. Further large-scale clinicopathologic studies are necessary to confirm the prognostic significance of tumor invasion depth in intestinal DLBCL.
Figures and Tables
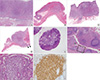 | Fig. 1Pathologic evaluation of depth of invasion and stage. Representative intestinal DLBCL cases involving mucosa and superficial submucosa (A, H&E, ×40), muscularis propria (B, H&E, scan view), subserosa (C, H&E, scan view), serosa (black arrows) (D, H&E, scan view), regional lymph node metastasis (E, H&E, ×40), and appendix non-contiguously (empty arrows) (F-H). (F) low magnification (H&E, ×40); (G) high magnification of region of interest in F (H&E, ×200); (H) CD20 immunostaining to identify the lesion (×200). |
 | Fig. 2Kaplan-Meier survival curves of overall survival according to clinical variables: (A) IPI risk group and (B) ECOG performance score. |
 | Fig. 3Kaplan-Meier survival curves of overall survival according to (A) T stage, (B) N stage, (C) M stage of the Paris classification system, and (D) modified Paris T stage. Note that the survival curves of the mucosa (M)/submucosa (SM) invasion group and the muscularis propria (PM) invasion group overlap completely. Abbreviations: M, mucosal confinement; PM, muscularis propria invasion; SM, submucosal invasion; SS, subserosal invasion; SI, serosa/adjacent organ invasion and/or perforation. |
Table 1

*Note that this is a conceptual category, and does not denote prognostically equivalent categories validated by survival analysis. †The Musshoff modified Ann Arbor stages do not take into account direct spread into adjacent tissues or organs, a state considered as stage IIE in the Lugano system. ‡The Paris M1 stage denotes multiple, non-contiguous involvements of the gastrointestinal tract, and is not represented in the modified Ann Arbor or Lugano stages.
Notes
References
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vzrdiman JW. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2008.
2. Gobbi PG, Ghirardelli ML, Cavalli C, Baldini L, Broglia C, Clò V, Bertè R, Ilariucci F, Carotenuto M, Piccinini L, et al. The role of surgery in the treatment of gastrointestinal lymphomas other than low-grade MALT lymphomas. Haematologica. 2000; 85:372–380.
3. Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011; 17:697–707.
4. Beaton C, Davies M, Beynon J. The management of primary small bowel and colon lymphoma: a review. Int J Colorectal Dis. 2012; 27:555–563.
5. Ruskone-Fourmestraux A, Delmer A, Hennequin C. Gastro-intestinal lymphomas. Gastroenterol Clin Biol. 2006; 30:2S81–2S90.
6. Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006; 7:379–391.
7. Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, Zeitz M, Riecken EO. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol. 2003; 21:2740–2746.
8. Lee J, Kim WS, Kim K, Ahn JS, Jung CW, Lim HY, Kang WK, Park K, Ko YH, Kim YH, et al. Prospective clinical study of surgical resection followed by CHOP in localized intestinal diffuse large B cell lymphoma. Leuk Res. 2007; 31:359–364.
9. Kim SJ, Kang HJ, Kim JS, Oh SY, Choi CW, Lee SI, Won JH, Kim MK, Kwon JH, Mun YC, et al. Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood. 2011; 117:1958–1965.
10. Musshoff K. Clinical staging classification of non-Hodgkin's lymphomas (author's transl). Strahlentherapie. 1977; 153:218–221.
11. Radaszkiewicz T, Dragosics B, Bauer P. Gastrointestinal malignant lymphomas of the mucosa-associated lymphoid tissue: factors relevant to prognosis. Gastroenterology. 1992; 102:1628–1638.
12. Rohatiner A, d'Amore F, Coiffier B, Crowther D, Gospodarowicz M, Isaacson P, Lister TA, Norton A, Salem P, Shipp M, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol. 1994; 5:397–400.
13. Ruskoné-Fourmestraux A, Dragosics B, Morgner A, Wotherspoon A, De Jong D. Paris staging system for primary gastrointestinal lymphomas. Gut. 2003; 52:912–913.
14. Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978; 42:693–707.
15. Koch P, del Valle F, Berdel WE, Willich NA, Reers B, Hiddemann W, Grothaus-Pinke B, Reinartz G, Brockmann J, Temmesfeld A, et al. Primary gastrointestinal non-Hodgkin's lymphoma: I. anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol. 2001; 19:3861–3873.
16. Chuang SS, Ye H, Du MQ, Lu CL, Dogan A, Hsieh PP, Huang WT, Jung YC. Histopathology and immunohistochemistry in distinguishing Burkitt lymphoma from diffuse large B-cell lymphoma with very high proliferation index and with or without a starry-sky pattern: a comparative study with EBER and FISH. Am J Clin Pathol. 2007; 128:558–564.
17. Bellan C, Stefano L, Giulia de F, Rogena EA, Lorenzo L. Burkitt lymphoma versus diffuse large B-cell lymphoma: a practical approach. Hematol Oncol. 2010; 28:53–56.
18. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004; 103:275–282.
19. Greene FL, Page DL, Fleming ID. AJCC cancer staging manual: TNM classification of malignant tumors. 6th ed. New York: Springer-Verlag;2002.
20. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging handbook. 7th ed. New York: Springer;2010.
21. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981; 47:207–214.
22. Kako S, Oshima K, Sato M, Terasako K, Okuda S, Nakasone H, Yamazaki R, Tanaka Y, Tanihara A, Kawamura Y, et al. Clinical outcome in patients with small-intestinal non-Hodgkin lymphoma. Leuk Lymphoma. 2009; 50:1618–1624.
23. Ferrucci PF, Zucca E. Primary gastric lymphoma pathogenesis and treatment: what has changed over the past 10 years? Br J Haematol. 2007; 136:521–538.
24. Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008; 19:1992–1999.
25. Nakamura S, Matsumoto T, Iida M, Yao T, Tsuneyoshi M. Primary gastrointestinal lymphoma in Japan: a clinicopathologic analysis of 455 patients with special reference to its time trends. Cancer. 2003; 97:2462–2473.
26. Shimodaira M, Tsukamoto Y, Niwa Y, Goto H, Hase S, Hayakawa T, Nagasaka T. A proposed staging system for primary gastric lymphoma. Cancer. 1994; 73:2709–2715.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download