This article has been corrected. See "Erratum: Correction of Acknowledgement. Establishment of an Orthotopic Mouse Non-Muscle Invasive Bladder Cancer Model Expressing the Mammalian Target of Rapamycin Signaling Pathway" in Volume 29 on page 617.
Abstract
We established an orthotopic non-muscle invasive bladder cancer (NMIBC) mouse model expressing the mammalian target of the rapamycin (mTOR) signaling pathway. After intravesical instillation of KU-7-lucs (day 0), animals were subsequently monitored by bioluminescence imaging (BLI) on days 4, 7, 14, and 21, and performed histopathological examination. We also validated the orthotopic mouse model expressing the mTOR signaling pathway immunohistochemically. In vitro BLI photon density was correlated with KU-7-luc cell number (r2 = 0.97, P < 0.01) and in vivo BLI photon densities increased steadily with time after intravesical instillation. The tumor take rate was 84.2%, formed initially on day 4 and remained NMIBC up to day 21. T1 photon densities were significantly higher than Ta (P < 0.01), and histological tumor volume was positively correlated with BLI photon density (r2 = 0.87, P < 0.01). The mTOR signaling pathway-related proteins were expressed in the bladder, and were correlated with the western blot results. Our results suggest successful establishment of an orthotopic mouse NMIBC model expressing the mTOR signaling pathway using KU-7-luc cells. This model is expected to be helpful to evaluate preclinical testing of intravesical therapy based on the mTOR signaling pathway against NMIBC.
Bladder cancer is the fourth most common cancer and the second most frequently diagnosed urologic cancer in men (1). Bladder cancer occurs as non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer according to disease severity, whereas > 70% of bladder cancer is NMIBC (2). Transurethral resection is the mainstay surgical treatment for NMIBC with an 80% success rate at the early stage; however, nearly 70% of patients suffer from tumor recurrence within 5 yr (3). Although the therapeutic protocols currently available for NMIBC, including intravesical bacillus Calmette-Guerin, have delayed progression, many patients with NMIBC experience disease progression with extended follow-up (4). Thus, there is a need to develop novel chemotherapeutic strategies to prevent disease progression and recurrence, and better knowledge about aberrant activation of cell signaling pathways that are involved in NMIBC to identify novel molecular targets.
One such target, the phosphatidylinositol 3'-kinase (PI3K) pathway, activates a number of signaling molecules, and the Akt/mammalian target of the rapamycin (mTOR) pathway is of particular interest because of its role inhibiting apoptosis and promoting cell proliferation (5). mTOR controls protein synthesis by phosphorylating downstream substrates, including p70S6 kinase (p70S6K) and eukaryotic initiation factor (eIF) 4E binding protein 1 (4E-BP1) (6). The PI3K/Akt/mTOR signaling pathway is linked to NMIBC, and its downstream effectors are associated with a high level of disease recurrence and progression as well as poor cancer-specific survival in vitro and in vivo (7, 8). Consequently, establishing a standard NMIBC animal model could contribute to research about the novel chemotherapeutic strategies related to the PI3K/Akt/mTOR signaling pathway to prevent recurrence and prevent the disease.
Orthotopic animal models enable a faster and clinically accurate tumor model to be obtained that is useful to better approximate human tumor cells (9). The use of immunodeficient mice in bladder cancer research has allowed direct implantation of human carcinomas into the corresponding organ of the animal model. Furthermore, advances in small animal imaging, such as bioluminescence imaging (BLI), have improved the capability to follow cancer progression non-invasively in vivo. BLI has the potential to become a valuable tool for early detection of tumor growth, and the orthotopic NMIBC model with BLI is optimum to evaluate various intravesical therapy methods (10). In this study, we established an orthotopic NMIBC mouse model expressing the mTOR signaling pathway, augmented by the use of serial BLI for in vivo tumor assessment and preclinical testing of intravesical therapy based on the mTOR signaling pathway against NMIBC.
Twenty 7-week-old female nude (nu/nu) mice were provided by Orient Bio (Seongnam, Korea). Animals spent a 1 week acclimation period under routine laboratory conditions before starting the experiments. All animals were housed in cages containing five animals and kept on a daily 12 hr light/dark cycle. Mice were fed a standard balanced diet and water ad libitum. The animal study was carried out according to a protocol approved by the Institutional Animal Care and Use Committee (NCC-12-178).
The KU-7 human bladder cancer cell line, engineered to stably express firefly luciferase and green fluorescent protein (KU-7-luc), was provided by Dr. HK Seo (National Cancer Center, Goyang, Korea). KU-7-luc was purchased from Caliper Life Sciences (Hopkinton, MA, USA). The in vitro study was performed using established human bladder cancer cell lines such as RT4, 253J, T24, 5637, TCCSUP cell lines, which were obtained from the Korean Cell Line Bank (Seoul, Korea). The six human bladder cell lines were used for Western blot. RT4 and T24 were cultured in McCoy's 5A Modified Medium (Gibco, Grand Island, NY, USA) with 10% fetal bovine serum (FBS). 253J and TCCSUP cells were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% FBS. The 5637 and KU-7-luc cells were cultured in Roswell Park Memorial Institute medium (Gibco), supplemented with 10% FBS. All media contained 50 µg/mL gentamycin sulfate, and all cell lines were maintained at 37℃ in 5% CO2.
The RT4, 253J, T24, 5637, TCCSUP, KU-7-luc cells were harvested, washed with PBS, and lysed in ice-cold RIPA lysis buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM PMSF, 5 mM aprotinin, leupeptin, and pepstatin) for 30 min. After protein quantitation with a BCA assay kit (Pierce, Rockford, IL, USA), 80 µg protein was boiled in sample loading buffer for 5 min before separation by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were transferred to a PVDF membrane, and blots were blocked with 5% non-fat dry milk in Tris-buffered saline with 0.1% Tween-20 (TBST) buffer (50 mM Tris-HCl, pH 7.4, 0.15 mM NaCl, 0.1% Tween-20) for 30 min at room temperature. The PVDF membranes (Millipore, Billerica, MA, USA) were incubated overnight at 4℃ with the indicated antibodies (Table 1). The blots were rinsed with TBST, incubated with a 1:3,000 dilution of goat anti-rabbit IgG antibody (Pierce), which had been conjugated with horseradish peroxidase for 1 hr at room temperature, and washed three times in TBST buffer for 10 min. The transferred proteins were visualized with a SuperSignal West Dura detection kit (Pierce) and exposed to the ChemiDocTMXRS system (Bio-Rad, Hercules, CA, USA).
Nineteen female nude (nu/nu) mice at 7-weeks-of-age (Orient Bio) were anaesthetized with isofluranefor intravesical implantation of KU-7-luc cells. The bladder urothelium was chemically lesioned by injecting 100 µL of poly-L-lysine (PLL; Sigma, St. Louis, MO, USA) into the bladder of each animal through a 24-gauge catheter (B/BrAUN, Melsungen, Germany). Bladders were washed with PBS and subsequently instilled with KU-7-luc cells (2.0×106) suspended in 50 µL PBS via a catheter. The cells were retained in the bladder for 2 hr by tying off the urethral orifice (Fig. 1A).
KU-7-luc cells were imaged using an IVIS 200 (Xenogen Corp., Alameda, CA, USA). Cultured cells were imaged in 24-well plates (BD Falcon, Franklin Park, NJ, USA) 10 min after adding D-luciferin (0.15 mg/mL, Invitrogen, Carlsbad, CA, USA) to the growth medium. Images were acquired and analyzed using Living Image software ver. 2.50 (Xenogen). Regions of interest (ROIs) were defined manually over cells to quantify signal intensities.
In vivo BLI of KU-7-luc cells was evaluated 10 min after intraperitoneal administration of 150 mg/kg D-luciferin (Invitrogen) on days 4, 7, 14, and 21, respectively after tumor cell implantation. The bioluminescence signal was acquired and analyzed using Living Image software ver. 2.50. ROIs were defined manually to encompass the bladder and quantify signal intensity (Fig. 1A).
We killed five mice with suspicion of bladder cancer as detected by BLI. The bladders were harvested and opened in the sagittal plane. After gross examination, the bladders were fixed in 4% paraformaldehyde, routinely processed and paraffin embedded, and stained with hematoxylin and eosin (H&E). We evaluated tumor stage by observing histological staining through a microscope (Fig. 1B). Bladder slices were sectioned into 5 µm sections using a microtome and stained with H&E for tumor volume measurements (largest width2×largest length×0.5).
Tissues were then stained in one batch following the immunohistochemistry protocol. The sections were heated in a microwave in 10 mM citric buffer for 20 min for antigen retrieval. The sections were incubated with the antibodies shown Table 1 overnight at 4℃. Table 1 lists all pertinent markers, including the vending, clone, dilution, pretreatment, and incubation conditions. The bound antibodies were visualized using Polink-2 plus HRP rabbit with an AEC kit (Golden Bridge International, Inc, Mukilteo, WA, USA).
SPSS software package ver. 20 (SPSS, Inc., Chicago,IL, USA) was used for all statistical analyses. One-way analysis of variance was used to detect significant differences between the groups, and Pearson's correlation test was used to study associations among parameters. A P< 0.05 was considered significant.
Fig. 2A shows a representative result of the linear proportional relationship between the in vitro BLI photon densities emitted from the cells at each cell number. The photon densities at cell numbers of 1×105, 1×106, and 3×106 were (1.32±0.08)×107, (1.59±0.22)×107, and (3.4±0.07)×107 photons/s respectively, and there was a good correlation between bioluminescence and cell number (r2=0.97, P<0.01). In vivo BLI photon densities were evaluated on days 4, 7, 14, and 21 days after the KU-7-luc cells were instilled, and the graph representing bioluminescence increased steadily with time (Fig. 2B).
Overall, 16 of 19 mice (84.2%) detected by BLI developed bladder cancer; and one mouse died 5 days after insillation of tumor cells into the bladder. In all mice, bladder cancers were detected on BLI before any clinical signs (hematuria or palpable tumor) developed. Table 2 shows the histological stage of bladder cancer in the mice models at each time point. The first tumor seen was stage Ta at 4 days, and the tumors progressed into T1 stage at 14 days. T1 stage tumors were predominant at 21 days but no muscle invasive bladder cancer was seen.
Fig. 2C shows the BLI photon densities according to bladder cancer stage, and the photon densities of T1 ([67.7±23.5]×107 photons/s) were higher than those of Ta ([9.8±1.5]×107 photons/s) significantly (P<0.01). Fig. 2D presents the BLI photon densities associated with tumor volume. When tumor volume was 0.72, 2.74, and 7.07 µL, photon intensities were 0.3×107, 21.0×107, and 81.1×107 photons/s, respectively, and histological tumor volume was positively correlated with BLI photon density (r2=0.87, P<0.01).
As shown in Fig. 3A, mTOR signaling pathway-related protein expression of the KU-7-luc cell line for mTOR, phosphorylated mTOR (p-mTOR), p70S6K, p-p70S6K, 4E-BP1, p-4E-BP1, eIF4E, and p-eIF4E were well observed. These results were consistent with those for the other bladder cancer cell lines (RT4, 253J, T24, 5637, and TCCSUP).
To determine the difference in mTOR signaling pathway-related protein expression between the in vitro and in vivo bladder cancer model using KU-7-luc cells, we examined the immunohistochemistry of the mTOR signaling pathway-related proteins in mouse tumor samples. The expression patterns of mTOR signaling pathway-related proteins on immunohistochemical staining from the orthotopic mouse NMIBC model using KU-7-luc cells was similar to that of the western blot with KU-7-luc cells.
Animal cancer models are important to evaluate the effects of different therapeutic interventions. A suitable bladder cancer model should resemble the human disease in both histology and behavior. Thus, the tumor should grow intravesically and should consist of pure urothelial cancer cells. Furthermore, NMIBCs are preferable; and establishing the model should be technically easy. The orthotopic bladder cancer model resembles the human situation resulting from seeding various tumor cells into the bladder mucosa. To enable preclinical testing of intravesical therapies based on the mTOR signaling pathway against NMIBC, we validated an orthotopic mice bladder cancer model, and augmented the model by serial BLI for in vivo tumor assessments. Our overall tumor establishment with 2.0×106 KU-7-Luc cells was >80% in mice at the scheduled time of 4-21 days. Moreover, we confirmed that the established orthotopic NMIBC model showed similar mTOR signaling pathway expression to that in the urothelial cancer cell lines.
Instillation of human cancer cell lines into the murine bladder can be approached by several methods. One of the approaches consists of directly injecting of tumor cells into the bladder wall after exposing the bladder with an abdominal incision (11-13). Although this technique result in almost 100% tumor take for specific cell lines, it requires an open surgical procedure and does not correctly reflect the NMIBC behavior. The tumors usually extend beyond the superficial layer through the normal urothelium and, thus, are not suitable for intravesical therapy for NMIBC. Transurethral administration of cancer cells with retrograde catheterization allowing for tumor deposition and seeding onto the mouse urothelium more closely reproduces NMIBC (10, 14-16). However, tumor take after transuretransurethral inoculation is cell line dependent and less reliable than that after intramural injection, so previous methods to enhance tumor take rate have involved various pretreatments of the mice bladder mucosa with specific agents (10).
We used poly-L-lysine (PLL) as a pretreatment agent instead of trypsin. Tumor take rates were 80%-100% with trypsin in a previous study, but the procedure-related death approached nearly 30%. Prolonged anesthesia and bladder perforation might be reasons for the high procedure-related mortality (17, 18). PLL is a cationic polypeptide used in a wide array of molecular and cell biology applications, such as gene transfer, enhancing biocompatibility of engraftment and for maintenance of poorly adhesive cells in an adhesive state. PLL enhances the electrostatic interaction between tumor cells and the bladder mucosa. After instilling 0.1 mL of 0.1 mg/mL PLL (molecular weight 70,000-150,000) for 20 min before implanting the cancer cells, one center reported a 94% tumor take rate (19). Tumor take using pretreatment with PLL is not inferior to pretreatment with trypsin and is tolerated well without significant adverse reactions or mortality of subjects.
Several imaging techniques (ultrasound, magnetic resonance imaging [MRI], or optical imaging) have been introduced in preclinical and clinical settings to assess presence, real-time growth, invasion, and metastasis of malignant tumor cells. Although MRI is feasible for detection and treatment monitoring of early stage orthotopic murine tumors, it does not offer an accurate diagnosis of small early lesions (<1 mm in diameter) due to its spatial resolution (20, 21). Furthermore, MRI devices are expensive and are not available in every center. Intravesical ultrasonography and cystoscopy provide a limited tumor stage positive-predictive ratio and low-throughput for following tumors after intravesical treatment in the orthotopic model (22, 23).
Whole-body optical imaging (BLI) allows repeated, real-time in vivo monitoring of tumor growth in small laboratory animals. BLI provides the most sensitive molecular imaging technique to date and does not require expensive and complicated technology. In addition, BLI measures only viable cells, an important beneficial feature to assess the real-time efficacy of a therapeutic agent (24). BLI has the potential to become a valuable tool for the early detection of tumor growth such as NMIBC. In our study, BLI first detected tumors on day 4 after tumor cell implantation, which was similar to published studies (10, 25). We validated the orthotopic murine model of bladder cancer in which tumor burden was longitudinally quantified by BLI and histology was used to measure tumor volume and stage. Histological tumor volume was significantly correlated with bioluminescence photon density, and a significant correlation was observed between photon density and histological tumor stage.
We also investigated activation status of the mTOR signaling pathway to validate whether serial manipulations and treatments of the orthotopic bladder cancer mice model might affect mTOR signaling pathway activation status. The ideal orthotopic cancer model for intravesical treatment must correctly reflect the characteristics of human cancer cell lines but there are no valid reports on this issue. First, we confirmed mTOR signaling pathway-related protein expression in the KU-7-luc cell line by expressing p-mTOR, mTOR, p70S6K, p-p70S6K, 4E-BP1, p-4E-BP1, eIF4E, and p-eIF4E. Expression of these proteins was consistent with the other bladder cancer cell lines, and immunohistochemical stains from established orthotopic bladder models showed similar expression of mTOR signaling pathway-related proteins in the KU-7-luc cancer cell line. We suggest that our procedures for establishing the orthotopic mouse NMIBC model did not significantly affect activation of the mTOR signaling pathway and our results indicate that our model is suitable for experiments and investigations of the mTOR pathway in vivo. In our knowledge, this study is the first study to establish orthotopic superficial NMIBC animal model confirmed by BLI, and to show activated mTOR pathway.
The first potential issue was that KU-7-luc cells exhibited an aggressive growth pattern and occasional multifocality. However, when the KU-7-luc cells were instilled into the mice bladder, they were generally confined to the lamina propria for up to 4 weeks after instillation and were correlated with the T1G2 stage of human bladder carcinoma (10). Xiao et al. (26), described three stages of tumor growth such as early tumor establishment (1-13 days), mid stage intravesical progression (14-21 days), and advanced intravesical progression and extravesical spread (22-50 days). We compared in vivo bioluminescence with histology on days 4, 7, 14, and 21, and performed necropsy of five mice whenever tumors were detected. Tumors were first detected on day 4 but only stages Ta and T1 were observed on day 21. No MIBC was found during the experiment.
Another potential issue may be a concern with this approach. A previous report suggested the possibility of intra-abdominal and/or extravesical tumor spread, renal and urethral tumor growth using a transurethral inoculation method for tumor cells (10). Extravesical tumor spread is related to traumatic catheterization resulting in placement of the catheter outside of the bladder, either in the peritoneum or paravesical space. Renal tumors are related to prolonged over-distention of the bladder with resulting vesicourethral reflux (VUR) and subsequent intrarenal implantation of tumor cells, and decreased instillation volume of tumor cells in PBS prevents VUR in mice, which is the same volume (50 µL) we used. Urethral tumors can also occur after a transurethral tumor injection followed by inadequate immobilization of the mice during tumor inoculation, which is related to inappropriate duration and depth of anesthesia. Thus, close attention is needed to avoid these problems, and all subjects should have appropriate tumor take.
Although we had these potential issues, our results suggest that we successfully established an orthotopic mouse NMIBC model expressing the mTOR signaling pathway using KU-7-luc cells, and that this model will be helpful to evaluate preclinical testing of intravesical therapy based on mTOR signaling against NMIBC.
Figures and Tables
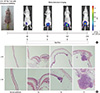 | Fig. 1Serial bioluminescence imaging and histopathological findings in the mice bladder after intravesical instillation of Ku-7-luc cells. (A) Schematic summary of the investigation. (B) Microscopic appearance of the KU-7-luc bladder tumor after hematoxylin and eosin staining of each stage groups taken randomly on days 4, 7, 14, and 21. |
 | Fig. 2×40 and ×100 magnification. Bioluminescence imaging of KU-7-luc tumors in vitro and in vivo. (A) Correlation between KU-7-luc cells and in vitro bioluminescence.Cells were serially diluted in a 96-well plate starting with 1×104 cell/well. Luciferin (0.15 mg/mL) was added to the wells, and the plate was imaged for 30 sec in an IVIS 2000 systemafter 15 min. Experiments were done in quadruplicate. (B) In vivo imaging of tumor growth over time. After intravesical instillation of 2×106 Ku-7-Luc cells on day zero, mice were imaged at 4, 7, 14, and 21 days. (C) Comparison of bioluminescence according to histological tumor stage. Error bars indicated standard error. (D) Correlation between bioluminescence and histological tumor volume. Bioluminescence is quantified in photons/s, n = 19. †P < 0.05, ‡P < 0.01. |
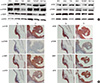 | Fig. 3mTOR signaling pathway related protein expression in human bladder cancer cell lines and the orthotopic mouse non-muscle invasive bladder cancer model using KU-7-luc cells. (A) Western blot analysis to evaluate the expression of phosphorylated-mTOR (p-mTOR), mTOR, p-70S6K, p70S6K, p-4EBP1, 4EBP1, p-eIF4E, and eIF4E in the human bladder cancer cell lines. (B) Immunohistochemical staining for p-mTOR, mTOR, p70S6K, p-p70S6K, 4E-BP1, p-4E-BP1, eIF4E, p-eIF4E in the orthotopic mouse non-muscle invasive bladder cancer model using KU-7-luc cells. mTOR, mammalian target of the rapamycin; p70S6K, p70S6 kinase; 4E-BP1, eukaryotic initiation factor 4E binding protein 1; eIF4E, eukaryotic initiation factor 4E. |
Notes
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2012R1A1A1002559). This study was also supported by the Biomedical Science Scholarship Grants, Department of Medicine, Chung-Ang University in 2013.
References
1. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.
2. Skinner E. Will a new intravesical chemotherapy agent improve the treatment of non-muscle-invasive bladder cancer? Nat Clin Pract Urol. 2007; 4:248–249.
3. Gasión JP, Cruz JF. Improving efficacy of intravesical chemotherapy. Eur Urol. 2006; 50:225–234.
4. Davis JW, Sheth SI, Doviak MJ, Schellhammer PF. Superficial bladder carcinoma treated with bacillus Calmette-Guerin: progression-free and disease specific survival with minimum 10-year followup. J Urol. 2002; 167:494–500.
5. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999; 13:2905–2927.
6. Isotani S, Hara K, Tokunaga C, Inoue H, Avruch J, Yonezawa K. Immunopurified mammalian target of rapamycin phosphorylates and activates p70 S6 kinase alpha in vitro. J Biol Chem. 1999; 274:34493–34498.
7. Seager CM, Puzio-Kuter AM, Patel T, Jain S, Cordon-Cardo C, Mc Kiernan J, Abate-Shen C. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res (Phila). 2009; 2:1008–1014.
8. Park SJ, Lee TJ, Chang IH. Role of the mTOR pathway in the progression and recurrence of bladder cancer: an immunohistochemical tissue microarray study. Korean J Urol. 2011; 52:466–473.
9. Soloway MS. Intravesical and systemic chemotherapy of murine bladder cancer. Cancer Res. 1977; 37:2918–2929.
10. Hadaschik BA, Black PC, Sea JC, Metwalli AR, Fazli L, Dinney CP, Gleave ME, So AI. A validated mouse model for orthotopic bladder cancer using transurethral tumour inoculation and bioluminescence imaging. BJU Int. 2007; 100:1377–1384.
11. Chang SG, Kim JI, Jung JC, Rho YS, Lee KT, An Z, Wang X, Hoffman RM. Antimetastatic activity of the new platinum analog [Pt(cis-dach) (DPPE). 2NO3] in a metastatic model of human bladder cancer. Anticancer Res. 1997; 17:3239–3242.
12. Jiang F, Zhou XM. A model of orthotopic murine bladder (MBT-2) tumor implants. Urol Res. 1997; 25:179–182.
13. Kleinerman DI, Dinney CP, Zhang WW, Lin SH, Van NT, Hsieh JT. Suppression of human bladder cancer growth by increased expression of C-CAM1 gene in an orthotopic model. Cancer Res. 1996; 56:3431–3435.
14. Bonfil RD, Russo DM, Schmilovich AJ, Garcia-Palazzo IB. Intravesical therapy with vinorelbine tartrate: antitumor activity in orthotopic murine cell carcinoma of the bladder. J Urol. 1997; 158:912–915.
15. Günther JH, Jurczok A, Wulf T, Brandau S, Deinert I, Jocham D, Böhle A. Optimizing syngeneic orthotopic murine bladder cancer (MB49). Cancer Res. 1999; 59:2834–2837.
16. Werthman PE, Drazan KE, Rosenthal JT, Khalili R, Shaked A. Adenoviral-p53 gene transfer to orthotopic and peritoneal murine bladder cancer. J Urol. 1996; 155:753–756.
17. Watanabe T, Shinohara N, Sazawa A, Harabayashi T, Ogiso Y, Koyanagi T, Takiguchi M, Hashimoto A, Kuzumaki N, Yamashita M, et al. An improved intravesical model using human bladder cancer cell lines to optimize gene and other therapies. Cancer Gene Ther. 2000; 7:1575–1580.
18. Chan ES, Patel AR, Smith AK, Klein JB, Thomas AA, Heston WD, Larchian WA. Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. J Urol. 2009; 182:2926–2931.
19. Mangsbo SM, Ninalga C, Essand M, Loskog A, Tötterman TH. CpG therapy is superior to BCG in an orthotopic bladder cancer model and generates CD4+ T-cell immunity. J Immunother. 2008; 31:34–42.
20. Chin J, Kadhim S, Garcia B, Kim YS, Karlik S. Magnetic resonance imaging for detecting and treatment monitoring of orthotopic murine bladder tumor implants. J Urol. 1991; 145:1297–1301.
21. Kikuchi E, Xu S, Ohori M, Matei C, Lupu M, Menendez S, Koutcher JA, Bochner BH. Detection and quantitative analysis of early stage orthotopic murine bladder tumor using in vivo magnetic resonance imaging. J Urol. 2003; 170:1375–1378.
22. Satoh H, Morimoto Y, Arai T, Asanuma H, Kawauchi S, Seguchi K, Kikuchi M, Murai M. Intravesical ultrasonography for tumor staging in an orthotopically implanted rat model of bladder cancer. J Urol. 2007; 177:1169–1173.
23. Arentsen HC, Hendricksen K, Oosterwijk E, Witjes JA. Experimental rat bladder urothelial cell carcinoma models. World J Urol. 2009; 27:313–317.
24. Van der Horst G, van Asten JJ, Figdor A, van den Hoogen C, Cheung H, Bevers RF, Pelger RC, van der Pluijm G. Real-time cancer cell tracking by bioluminescence in a preclinical model of human bladder cancer growth and metastasis. Eur Urol. 2011; 60:337–343.
25. Jurczok A, Fornara P, Soling A. Bioluminescence imaging to monitor bladder cancer cell adhesion in vivo: a new approach to optimize a syngeneic, orthotopic, murine bladder cancer model. BJU Int. 2008; 101:120–124.
26. Xiao Z, McCallum TJ, Brown KM, Miller GG, Halls SB, Parney I, Moore RB. Characterization of a novel transplantable orthotopic rat bladder transitional cell tumour model. Br J Cancer. 1999; 81:638–646.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download