Abstract
Figures and Tables
Fig. 1
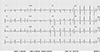
Fig. 2
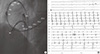
Fig. 3
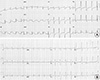
Fig. 4

Journal List > J Korean Med Sci > v.29(2) > 1022477




Sehyo Yune 
https://orcid.org/http://orcid.org/0000-0002-9223-3586
Woo Joo Lee 
https://orcid.org/http://orcid.org/0000-0002-4790-3560
Ji-won Hwang 
https://orcid.org/http://orcid.org/0000-0002-7098-3546
Eun Kim 
https://orcid.org/http://orcid.org/0000-0001-9401-7388
Jung Min Ha 
https://orcid.org/http://orcid.org/0000-0002-0075-4449
June Soo Kim 
https://orcid.org/http://orcid.org/0000-0001-7569-6450