Abstract
It has not yet been determined whether chronic exposure to relatively low doses of pioglitazone increases risk of bladder cancer. We aimed to assess the risk of bladder cancer associated with pioglitazone in Korean patients. This was a retrospective cohort study of diabetic patients who had ≥ 2 clinic visits between November 2005 and June 2011 at one of four tertiary referral hospitals in Korea. A prevalent case-control analysis nested within the cohort was conducted to further adjust confounders. A total of 101,953 control patients and 11,240 pioglitazone-treated patients were included, in which there were 237 and 30 cases of incidental bladder cancer (64.9 and 54.9 per 100,000 person-years; age, sex-adjusted HR 1.135, 95% confidence interval [CI] 0.769-1.677), respectively. In the prevalent case-control analysis nested within the cohort, use of pioglitazone for a duration of > 6 months, but not ever use of pioglitazone, was associated with an increased rate of bladder cancer as compared to never use of pioglitazone. In conclusion, we failed to exclude the possible association between use of pioglitazone for a duration of > 6 months and bladder cancer.
It has previously been suggested that the use of pioglitazone can increase the risk of developing bladder cancer in a dose-response manner (1-5). In addition, several recent meta-analyses also supported this association (6-8). The significance of these meta-analyses, however, was primarily dependent on a large-scale study conducted in a French population. Although the French study had sufficient statistical power given its large number of study subjects, the data was not adjusted for specific risk factors for bladder cancer other than age, sex, and use of glucose-lowering agents (4). The exact mechanism of the association between pioglitazone and bladder cancer has not been fully understood, although a 'predisposing' capacity of peroxisome proliferator-activated receptor rather than genotoxic or carcinogenic property has been suggested (9-11).
In fact, the association between the use of pioglitazone and the risk of developing bladder cancer has to date not been definitely demonstrated in an Asian population. Interestingly, studies conducted in Taiwanese and Japanese populations have suggested a non-significant increased risk of developing bladder cancer in short-term users of pioglitazone without a dose-dependent relationship (5, 12, 13). Moreover, use of pioglitazone at a dosage exceeding 15 mg per day for patients with diabetes has not been approved in Korea till 2013. The dosage of up to 15 mg per day is lower than that typically used in Europe and North America, and it has not yet been determined whether chronic exposure to low doses of pioglitazone exhibits a similar risk.
We aimed to assess the association between pioglitazone use and the development of bladder cancer in a cohort from four tertiary referral hospitals in Korea.
We extracted a clinical database of diabetic patients with two or more clinic visits between November 2005 and June 2011 from the electronic medical records of four tertiary referral hospitals in Korea (Samsung Medical Center, Severance Hospital, Asan Medical Center, and Seoul St. Mary's Hospital, Seoul, Korea). Patients were excluded from the study if they had a diagnosis of bladder cancer prior to or within three months of the start of the study period.
Use of pioglitazone was defined as receipt of two prescriptions for pioglitazone within 6 months of each other during the study period. Duration of therapy was calculated according to the interval between the first and last prescriptions. Therefore, the duration of therapy was a minimum of the actual duration, as additional medication may have been administered by another institution prior to visits conducted at the study hospitals. For this reason, we conducted a prevalent case-control analysis in parallel. In the prevalent case-control analysis, the duration of therapy was categorized as never use, ever use, or >6 months based on the minimum actual duration confirmed by review of the medical record. Patients with bladder cancer were identified according to hospital discharge diagnosis (ICD-10 code C67).
From the source cohort, all patients with bladder cancer were identified. The index date was defined as the date of bladder cancer diagnosis. For each patient with a diagnosis of bladder cancer, three randomly selected control patients were matched according to age (±1 yr), sex, and the duration between cohort entry and either the diagnosis of bladder cancer or the end of follow-up (±3 months). The index date for the controls was the date of cohort entry. The risk ratios were estimated from the odds ratios obtained from the prevalent case-control analysis, based on the rarity-assumption.
Comparisons of categorical and continuous variables between pioglitazone-treated and control patients were performed using chi-square tests and t-tests, respectively. The crude incidence rates of bladder cancer for pioglitazone-treated and control patients were calculated by dividing the number of bladder cancer cases by the overall follow-up in terms of person-years. Cox proportional hazards models were used to generate adjusted hazard ratios (HRs) to compare the incidence rates of bladder cancer between pioglitazone-treated and control patients. The covariates included age at the time of cohort entry and sex. Cox proportional hazards models were constructed for all study subjects. In parallel, a conditional logistic regression was also performed to adjust for potential confounders among the matched subjects for prevalent case-control analysis. The confounders adopted as covariates in the prevalent case-control analysis included smoking status, urinary tract disease or symptoms of a urinary tract infection or urolithiasis, duration of diabetes, fasting blood glucose levels, congestive heart failure, estimated glomerular filtration rate, history of other malignancy, and anti-diabetic drugs other than pioglitazone. These were based on established conventional parameters that have previously been correlated with either bladder cancer or the choice of pioglitazone therapy as a part of diabetes treatment. The covariates were collected based on the subject's status at index date on medical record.
All patients in whom the duration of observation was less than three months were also excluded. All study protocols were approved by the institutional review board (IRB) of each hospital that participated in the study (IRB No. 2012-06-083 in Samsung Medical Center, No. 4-2011-0646 in Severance Hospital, No. 2012-0046 in Asan Medical Center, and No. KC12RIMI0037 in Seoul St. Mary's Hospital). Informed consent was waived by the IRB.
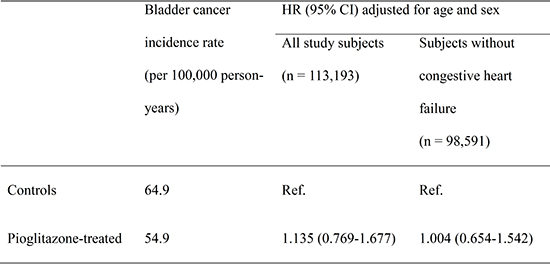
After exclusion of patients with a diagnosis of bladder cancer prior to or within 3-months of entry into the cohort as well as patients with a duration of observation of <3 months, 113,193 patients with type 2 diabetes were included in this study. Of these patients, 11,240 patients had a history of pioglitazone treatment (Table 1).
A total of 237 (0.23%) and 30 (0.27%) cases of bladder cancer were identified in control and pioglitazone-treated subjects during the follow-up period of 370,620 and 54,650 person-years, respectively. The incidence rate of bladder cancer per 100,000 person-years was 64.9 and 54.9 in control and pioglitazone-treated subjects, respectively (Table 2). Use of pioglitazone use was not significantly associated with the incidence of bladder cancer (age, sex-adjusted HR 1.135, 95% confidence interval [CI] 0.769-1.677).
To further adjust confounders other than age and sex, a prevalent case-control analysis nested within the cohort was conducted. In this analysis, the duration of pioglitazone treatment was re-defined based on a detailed review of the medical record. Cases with bladder cancer (n=208) were matched to 620 control subjects based on their age, sex, and the duration of observation in the source cohort. On univariate analysis, a history of another malignancy, benign prostatic hyperplasia, low hemoglobin, low estimated glomerular filtration rate (eGFR), and high fasting plasma glucose were associated with bladder cancer (Table 3). Based on these results, congestive heart failure, duration of diabetes, smoking status, history of another malignancy, benign prostatic hyperplasia, hemoglobin level, eGFR, fasting plasma glucose (mg/dL), and anti-diabetic drugs other than pioglitazone were included in the multivariate analysis using conditional logistic regression analysis for covariates. In this analysis, use of pioglitazone for a duration of >6 months, but not ever use, was associated with an increased incidence rate of bladder cancer (adjusted rate ratio 1.969; 95% CI, 1.020-3.802; Table 4).
In our multi-center hospital-based cohort (n=113,193), the incidence of bladder cancer was similar to those in the previous population-based studies (1, 2, 4, 5). Although increase in bladder cancer incidence in pioglitazone-treated patients was not significant in Cox regression analysis, there was an association between >6 months of pioglitazone therapy and an increased risk of bladder cancer in the prevalent case-control analysis nested within the cohort in which confounders other than age and sex were adjusted and the duration of pioglitazone was re-defined.
No significant association between pioglitazone use and bladder cancer found on Cox regression analysis in this study is not surprising, because most previous studies including the studies conducted in the Kaiser Permanente Northern California (KPNC) cohort (2) and the Taiwanese cohort (5) did not demonstrated the significance of association between ever use of pioglitazone and increased incidence of bladder cancer. In the KPNC cohort, a significant association between pioglitazone use and bladder cancer was identified only after a detailed dose-response analysis (2). Notably, the significant association between ever use of pioglitazone therapy and the risk of bladder cancer in recent meta-analyses was primarily based on the results of large-scale French study (4, 6). In addition, the hazard ratio of Cox regression in this study could be underestimated because the study subjects in this study was not restricted to newly-diagnosed diabetes patients and the exposure definition did not include the use of pioglitazone outside the four hospitals included in this study. Therefore, the results of Cox regression in this study is not in contrast to the association between pioglitazone exposure and bladder cancer suggested by the previous studies, because the available evidence of such association to date was mostly from the delicate dose-response analysis rather than the comparison of ever and never use (1-3, 12). For this reason, we additionally conducted a prevalent case-control analysis nested within the source cohort to further adjust the confounders and to re-define the treatment duration of pioglitazone based on a detailed review of medical records.
Although no significant association between pioglitazone use and bladder cancer was found on Cox regression analysis in this study, we did observe a significant association in our prevalent case-control analysis when the observed duration of treatment was >6 months. The additional analysis of the subjects with >6 months of pioglitazone treatment was performed primarily because most of the previous large scale cohort studies excluded patients with an observed duration of treatment <6 months in order to exclude cases of latent bladder cancer that were not identified until the initiation of pioglitazone therapy (2, 4).
Although there have been multiple large-scale cohort studies that have described a dose-dependent relationship between pioglitazone and the risk of bladder cancer, most of this evidence was obtained from European and North American populations (1-3, 12). In a Taiwanese cohort, this dose-dependent relationship was not observed, as the majority of bladder cancer cases in pioglitazone users were found in those that underwent short-term therapy (5). A case-control study in Japan also suggested an association between the use of pioglitazone for <24 months and the risk of bladder cancer, while the overall hazard ratio was not significant (13). Additionally, in our preliminary case-control study conducted in patients at a single university hospital, a dose-dependent relationship was not identified (14). Although the delicate dose-response analysis was impossible, our results suggest that an association between long-term use of pioglitazone and the risk of bladder cancer cannot be excluded in the Korean population, given that patients treated with pioglitazone for >6 months in the current study are likely to have a longer actual duration of therapy.
Several limitations of the current study should also be noted, however. First, this is not a population-based study. Therefore, our conclusions should not be applied in the primary care setting in which the risk profile of the patient population for the bladder cancer may be different. Secondly, we were unable to analyze the exact duration and cumulative dose of pioglitazone due to our limited access to health information outside of the records from the four tertiary hospitals that participated in this study. Thirdly, subjects with bladder cancer in this study showed significantly higher frequencies of other malignancy, benign prostate hyperplasia, anemia, decreased renal function, and insulin resistance, and more insulin users, suggesting that worse medical condition even they were not older or male predominant. Therefore, the results could be biased by these factors, although we used these variables as covariates for multiple logistic analysis.
In conclusion, we failed to exclude the possible association between use of a 15 mg pioglitazone dose for a duration of >6 months and bladder cancer, although increase in bladder cancer incidence in pioglitazone-treated patients was not significant in Cox regression analysis. Until the results of a population-based study with a delicate dose-response analysis in Koreans are available, clinicians should carefully monitor the possible development of bladder cancer and consider the risk-to-benefit ratio before starting pioglitazone therapy in high risk patients.
Figures and Tables
Notes
References
1. Azoulay L, Yin H, Filion KB, Assayag J, Majdan A, Pollak MN, Suissa S. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. BMJ. 2012; 344:e3645.
2. Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP Jr, Vaughn DJ, Nessel L, Selby J, Strom BL. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011; 34:916–922.
3. Mamtani R, Haynes K, Bilker WB, Vaughn DJ, Strom BL, Glanz K, Lewis JD. Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J Natl Cancer Inst. 2012; 104:1411–1421.
4. Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012; 55:1953–1962.
5. Tseng CH. Pioglitazone and bladder cancer: a population-based study of Taiwanese. Diabetes Care. 2012; 35:278–280.
6. Colmers IN, Bowker SL, Majumdar SR, Johnson JA. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ. 2012; 184:E675–E683.
7. Ferwana M, Firwana B, Hasan R, Al-Mallah MH, Kim S, Montori VM, Murad MH. Pioglitazone and risk of bladder cancer: a meta-analysis of controlled studies. Diabet Med. 2013; 30:1026–1032.
8. Zhu Z, Shen Z, Lu Y, Zhong S, Xu C. Increased risk of bladder cancer with pioglitazone therapy in patients with diabetes: a meta-analysis. Diabetes Res Clin Pract. 2012; 98:159–163.
9. Guan YF, Zhang YH, Breyer RM, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptor gamma (PPARgamma) in human transitional bladder cancer and its role in inducing cell death. Neoplasia. 1999; 1:330–339.
10. Yoshimura R, Matsuyama M, Segawa Y, Hase T, Mitsuhashi M, Tsuchida K, Wada S, Kawahito Y, Sano H, Nakatani T. Expression of peroxisome proliferator-activated receptors (PPARs) in human urinary bladder carcinoma and growth inhibition by its agonists. Int J Cancer. 2003; 104:597–602.
11. Suzuki S, Arnold LL, Pennington KL, Kakiuchi-Kiyota S, Wei M, Wanibuchi H, Cohen SM. Effects of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on the urine and urothelium of the rat. Toxicol Sci. 2010; 113:349–357.
12. Tseng CH. Diabetes and risk of bladder cancer: a study using the National Health Insurance database in Taiwan. Diabetologia. 2011; 54:2009–2015.
13. Fujimoto K, Hamamoto Y, Honjo S, Kawasaki Y, Mori K, Tatsuoka H, Matsuoka A, Wada Y, Ikeda H, Fujikawa J, et al. Possible link of pioglitazone with bladder cancer in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2013; 99:e21–e23.
14. Song SO, Kim KJ, Lee BW, Kang ES, Cha BS, Lee HC. The risk of bladder cancer in Korean diabetic subjects treated with pioglitazone. Diabetes Metab J. 2012; 36:371–378.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download