Abstract
We aimed to investigate differences in clinical manifestations and outcomes between adult and child patients with Henoch-Schönlein purpura (HSP), and to analyze the factors associated with poor prognosis for HSP nephritis. This retrospective 10-yr study enrolled 160 patients with HSP who visited Severance Hospital. Purpura was mostly detected in lower extremities, but purpura in upper extremities was more frequently observed in adults than children (41.7% vs 19.3%). Children had a greater frequency of arthralgia (55.4% vs 27.1%), while adults had a greater frequency of diarrhea (20% vs 1.6%). Anemia, elevated C-reactive protein, and level of IgA were more frequently observed in adults (25% vs 7.1%, 65.6% vs 38.4%, 26.3% vs 3.5%). Renal involvement in adults was more severe than in children (79.2% vs 30.4%). Chronic renal failure showed a significant difference in outcomes of HSP between adults (10.4%) and children (1.8%) after a follow up period of an average of 27 months. Furthermore, renal insufficiency at diagnosis was significantly related to the progression to chronic renal failure. Our results showed several differences in the clinical features of HSP between adults and children. Adults with HSP had a higher frequency of renal insufficiency and worse renal outcomes than children. Renal insufficiency at diagnosis might be of predictive value for the progression to chronic renal failure in HSP patients.
Henoch-Schönleinpurpura (HSP) is a systemic vasculitis involving small vessels with the deposition of immune complexes containing IgA (1). Clinical manifestations show purpuric skin lesions, arthralgia, abdominal pain, bleeding, nephritis, neurologic involvement, and pulmonary hemorrhage (2). Nephritis is a severe complication of HSP that can proceed to end stage renal failure, and its severity affects long term prognosis (3).
HSP primarily affects children, and its annual incidence is estimated to be 15 cases/100,000 per year (4) in children compared to 1.3 cases/100,000 per year in adults (5). HSP has been extensively studied in children but much less focus was given to adults.
Early studies in the 1970s and 1980s suggested a higher percentage with renal involvement with poorer renal outcomes in adults (6, 7), and the age at onset has been considered to be animportant factor for disease manifestation, severity and outcome. There are a few previous studies that have compared the disease spectrum as seen in adults with that seen in children (2, 8-12), but little has been known about its clinical manifestations and outcomes in adults until now.
This study was performed to investigate the differences in clinical parameters, laboratory data, and outcomes between adults and children with HSP, and to analyze the factors associated with poor prognosis for HSP nephritis.
We retrospectively evaluated the medical records of 160 patients with HSP who visited Severance Hospital, Yonsei University Health System in Seoul, Korea, between December 1999 and November 2009. The patients were classified into two groups according to their age at diagnosis of HSP (13): adults (N=48, >20 yr old) and children (N=112, ≤20 yr old). All patients fulfilled the European League against Rheumatism/Paediatric Rheumatology International Trials Organisation/Paediatric Rheumatology European Society (EULAR/PRINTO/PRES) criteria for HSP, which included having purpura and at least one of the four following phenomena: a) abdominal pain; b) histopathology, typically leukocytoclastic vasculitis or proliferative glomerulonephritis with IgA deposits; c) arthritis or arthralgia; and d) renal involvement (14). On the other hand, the American College of Rheumatology (ACR) criteria requires 2 of the following 4 phenomena: a) palpable purpura, non-thrombocytopenic; b) age ≤20 yr at disease onset; c) bowel angina; and d) histologic changes showing granulocytes in the walls of arterioles or venules (13). We utilized the EULAR/PRINTO/PRES criteria because; a) they do not include age criteria, and the age criteria of the ACR are not suitable for adults in our study; b) joint symptoms and renal involvement, which are main manifestations of HSP, are excluded in the ACR criteria; c) the criteria for adults have not been developed yet. We excluded patients with cutaneous vasculitis secondary to other collagen vascular disease. Predisposing factors for the development of HSP, including the previous upper respiratory tract infection (URI), were evaluated. Clinical manifestations such as skin, joint, gastro-intestinal, and renal involvement and outcomes such as chronic renal failure, persistence of hematuria, or proteinuria were assessed.
Patients were considered hypertensive if their blood pressure was greater than 130/80 mmHg in adults, greater than the 90th percentile values for height in children, or when receiving anti-hypertensive treatments. Renal insufficiency was defined as creatinine clearance <60 mL/min/1.73 m2 (calculated by the Modification of Diet in Renal Disease formula in adults and by the Schwartz formula in children). Nonnephrotic proteinuria was defined as proteinuria≥0.15 g/day, ≤3.5 g/day in adults and ≥4 mg/m2/hr, ≤40 mg/m2/hr in children. Nephrotic syndrome was defined as proteinuria >3.5 g/day in adults and >40 mg/m2/hr in children, serum albumin <2.5 g/dL, with or without edema. Microscopic hematuria was defined as >5 RBC/HPF, and gross hematuria was defined as >1,500 RBC/HPF. If patients complained of well-defined arthralgia or if arthritis was observed on examination, then they were considered to have joint involvement. Gastrointestinal involvement included nausea, vomiting, abdominal pain, diarrhea, and hematochezia.
Renal outcome at last follow up was assessed as complete recovery, relapse, persistent hematuria, persistent proteinuria, or progression to chronic renal failure. Relapse was considered when a patient who was diagnosed with HSP and who had been asymptomatic for at least 1 month presented a new flare of skin lesions or other systemic complications. Chronic renal failure was defined as creatinine clearance <60 mL/min/1.73 m2 for more than 3 months.
Continuous data were described as mean and standard deviation (mean±SD), and categorical variables as percentages. Chi-square test or Fisher's exact test was used to compare categorical variables. Binary logistic regression analysis was used to explore the independent prognostic factors for poor renal outcome. All differences were considered significant at P<0.05. Calculations were performed with SPSS (version 17.0; SPSS Inc., Chicago, IL, USA).
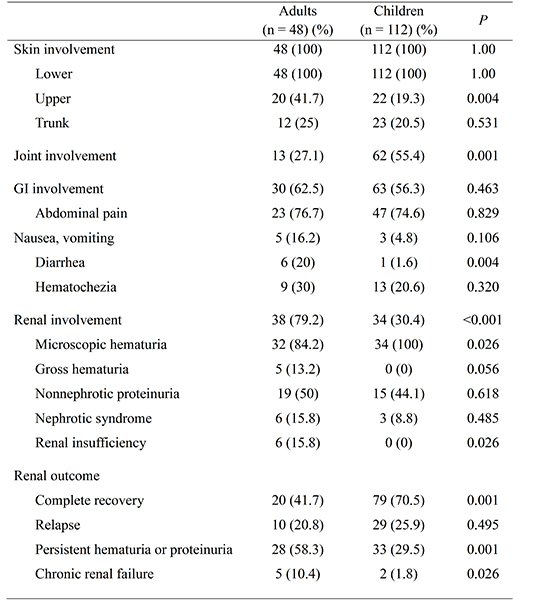
A total of 160 HSP patients were studied (48 adults, 112 children). Among them, 56.2% of the adults and 57.1% of the children were males. HSP was more frequent during the spring and winter in both adults (68.8%) and children (75.1%). URI was a common predisposing factor for both adults (22.9%) and children (36.6%), and it was not statistically different between adults and children. However, prior drug exposure (12.5%) and underlying cancer (10.4%) were identified as predisposing factors only in adults (Table 1). Drugs included non-steroidal anti-inflammatory drugs (NSAIDs), furosemide, warfarin, and anti-tuberculosis medications. Cancers were hepatocellular carcinoma, duodenal cancer, and gastric cancer.
Purpura was present in all patients. In both groups, purpura was mostly detected in the lower extremities, but purpura in the upper extremities was more frequently observed in adults than children. Joint involvement occurred less frequently in adults. The most frequent pattern observed was oligoarthritis affecting the ankles and/or knees. Gastrointestinal involvement did not differ between adults and children. The most common symptom was abdominal pain in both groups. Diarrhea presented more frequently in adults. Renal involvement presented in 79.2% of the adults and in 30.4% of the children, which was a significant difference. Microscopic hematuria and a somewhat mild renal manifestation, was more frequently observed in children. However, severe manifestations such as nephrotic syndrome and renal insufficiency occurred more frequently in adults.
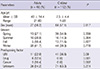
Anemia, elevated C-reactive protein, and an increased level of IgA were more frequently observed in adults. Increased platelets occurred more often in children (Table 2).
Immunosuppressant treatment was administered more often in adults than in children. The glucocorticoid treatment rate was not significantly different between two groups; however, cytotoxic drugs such as cyclosporine, cyclophosphamide, and azathioprine were prescribed more frequently in adults (12.5%) than in children (4.5%).
The period of follow up did not differ between the adults (mean 27.6 months) and children (mean 27.5 months). The renal outcome at last follow up was relatively worse in the adult group. The rate of complete recovery in the adult group was significantly lower than that in the childhood group (41.7% vs 70.5%). Persistent hematuria or proteinuria occurred more frequently in adults than in children (58.3% vs 29.5%), and progression to chronic renal failure presented more frequently in adults than in children (10.4% vs 1.8%) with significance. The relapse rate of the two groups did not differ (Table 3).
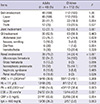
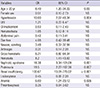
In univariate analysis, age >20 yr, hypertension, presence of abdominal pain, arthralgia, hematuria, proteinuria, nephrotic syndrome, renal insufficiency, and anemia were identified as prognostic factors for progression to chronic renal failure (Table 4). Multivariate analyses were derived from the risk factors detected by univariate analysis at P less than 0.05 (Table 5). Renal insufficiency at the time of the diagnosis was found to be an independent risk factor for progression to chronic renal failure (OR, 34; 95% CI, 3.70-312.09; P=0.002).
Our results showed several differences in clinical features and outcomes of HSP between adults and children. A slight male predominance both in adults and in children in our study was consistent with many previous studies (2, 8, 15-19). The incidence of HSP was quite a bit higher in spring and winter and URI was common in both adults and children in this study. Previous studies have shown that the incidence of HSP was highest in winter and URI was common (2, 8, 9, 15). This implies a possible infection etiology. HSP commonly follows URI, and viruses such as parvovirus B19 (20) and bacteria such as staphylococci (21), and streptococci (22) have been suggested as possible pathogens associated with HSP. Prior drug exposure and underlying malignancy as the predisposing factors were shown only in adults in this study. IgA immune complex deposition resulting in HSP can be caused by exposure to antigens from tumors, often adenocarcinomas (23); lymphomas, both non-Hodgkin's and Hodgkin's (23); and IgA myeloma (24). Mitsui et al. (25) reported that 24 patients were associated with malignancy among 103 HSP patients, and all of them were adults except for one patient. Malignancy might be a different etiologic factor between adults and children. The prior drug exposure was higher in adults than in children in previous studies (2, 8-10, 12). Quinolones and clarithromycin (26) have been described in association with HSP.
Purpura was detected in the lower extremities in all patients, consistent with previous studies (8, 15). However, purpura in the upper extremities was more frequently observed in adults than children, unlike the Spanish study (8). Joint involvement occurred more frequently in children than in adults in our study, while the frequency was similar in previous studies (2, 8-11). The common joint involvement pattern was similar with that reported in previous studies (2, 11, 18). The frequency and common symptom of gastrointestinal involvement were comparable with previous studies (2, 8-12). Diarrhea presented more frequently in adults, consistent with one study (9). HSP with renal involvement has been reported as 20%-28% in children, mostly limited to isolated hematuria or minimal proteinuria lasting less than 1 month in 70%-80% of patients (17), in contrast to 49%-83% in adults with poorer outcome (18, 25, 27). This tendency was also found in our study. Renal involvement presented in 79.2% of the adults and in 30.4% of the children. Microscopic hematuria, a rather mild renal manifestation, was more frequently observed in children, in contrast to severe manifestations such as nephrotic syndrome and renal insufficiency, which occurred more frequently in adults. Previous studies showed a similar tendency with our results (2, 8-10, 12). Anemia, elevated C-reactive protein, and an increased level of IgA were more frequently observed in adults. Increased platelets occurred more often in children. Laboratory findings were different from study to study and were contradictory (2, 8, 9, 12).
Progression to chronic renal failure was reported in 2%-15% in children (28, 29) and 8%-68% in adult HSP patients (18, 25, 27). The renal outcome at last follow up was worse in adult in this study. Complete recovery was observed more frequently in children than in adults. 10.4% of adults and 1.8% of the children progressed to chronic renal failure with statistical significance.
We found that older age, hypertension, presence of abdominal pain, arthralgia, hematuria, proteinuria, nephrotic syndrome, renal insufficiency, and anemia at the time of diagnosis were prognostic factors for progression to chronic renal failure by univariate analysis. Only initial renal insufficiency was found to be an independent risk factor for progression to chronic renal failure by multivariate analysis. Pillebout et al. (18) reported that initial renal insufficiency, proteinuria, and on renal biopsy, the degree of interstitial fibrosis, percentage of sclerotic glomeruli, and presence of glomeruli with fibrinoid necrosis were associated with a severe chronic renal failure. Coppo et al. (17) suggested that older age, being female, and mean proteinuria levels during follow up were risk factors for dialysis therapy but renal biopsy findings were not related. The risk factor, initial renal insufficiency, found in our study is in agreement with Pillebout et al.; however, although a renal biopsy was done only in 9 children and 21 adult patients in our study, the biopsy findings were not associated with progression to chronic renal failure, as reported by Coppo et al. Our study had a limitation in that the renal biopsy findings and mean proteinuria levels during follow-up were not performed by all patients.
So far, six studies have been published about Korean adult HSP (30-35). However, only two studies compared adult and children HSP patients (34-35), and one of the two focused their investigation on nephritis (34). The analysis by Hong et al. (35) was similar to ours; however, they did not find any statistically different findings except for renal involvement (Table 6). The differences in the number of enrolled adult patients with HSP between our study and the one by Hong et al.(35) may explain the discrepancy between these findings. We found statistically significant differences in clinical manifestations and outcomes between Korean adults and children with HSP. This study confirmed the observation of different manifestations and outcomes between adults and children, particularly in renal involvement. Adults with HSP had more frequent renal involvement and worse renal outcomes than children. Furthermore, this study identified renal insufficiency at the time of the diagnosis as an independent risk factor for progression to chronic renal failure. More attention and extended follow up will be required in adult HSP patients with renal involvement, especially in patients showing renal insufficiency at the time of diagnosis.
Figures and Tables
References
1. O'Donoghue DJ, Darvill A, Ballardie FW. Mesangial cell autoantigens in immunoglobulin A nephropathy and Henoch-Schönlein purpura. J Clin Invest. 1991; 88:1522–1530.
2. Blanco R, Martínez-Taboada VM, Rodríguez-Valverde V, García-Fuentes M, González-Gay MA. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum. 1997; 40:859–864.
3. Sano H, Izumida M, Shimizu H, Ogawa Y. Risk factors of renal involvement and significant proteinuria in Henoch-Schönlein purpura. Eur J Pediatr. 2002; 161:196–201.
4. Nielsen HE. Epidemiology of Schönlein-Henoch purpura. Acta Paediatr Scand. 1988; 77:125–131.
5. Fervenza FC. Henoch-Schönlein purpura nephritis. Int J Dermatol. 2003; 42:170–177.
6. Fillastre JP, Morel-Maroger L, Richet G. Schönlein-Henoch purpura in adults. Lancet. 1971; 1:1243–1244.
7. Lee HS, Koh HI, Kim MJ, Rha HY. Henoch-Schönlein nephritis in adults: a clinical and morphological study. Clin Nephrol. 1986; 26:125–130.
8. García-Porrúa C, Calviño MC, Llorca J, Couselo JM, González-Gay MA. Henoch-Schönlein purpura in children and adults: clinical differences in a defined population. Semin Arthritis Rheum. 2002; 32:149–156.
9. Uppal SS, Hussain MA, Al-Raqum HA, Nampoory MR, Al-Saeid K, Al-Assousi A, Abraham M, Malaviya AN. Henoch-Schönlein's purpura in adults versus children/adolescents: a comparative study. Clin Exp Rheumatol. 2006; 24:S26–S30.
10. Hung SP, Yang YH, Lin YT, Wang LC, Lee JH, Chiang BL. Clinical manifestations and outcomes of Henoch-Schönlein purpura: comparison between adults and children. Pediatr Neonatol. 2009; 50:162–168.
11. Ilan Y, Naparstek Y. Schönlein-Henoch syndrome in adults and children. Semin Arthritis Rheum. 1991; 21:103–109.
12. Lin SJ, Huang JL. Henoch-Schönlein purpura in Chinese children and adults. Asian Pac J Allergy Immunol. 1998; 16:21–25.
13. Mills JA, Michel BA, Bloch DA, Calabrese LH, Hunder GG, Arend WP, Edworthy SM, Fauci AS, Leavitt RY, Lie JT, et al. The American College of Rheumatology 1990 criteria for the classification of Henoch-Schönlein purpura. Arthritis Rheum. 1990; 33:1114–1121.
14. Ozen S, Pistorio A, Iusan SM, Bakkaloglu A, Herlin T, Brik R, Buoncompagni A, Lazar C, Bilge I, Uziel Y, et al. EULAR/PRINTO/PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008: part II: final classification criteria. Ann Rheum Dis. 2010; 69:798–806.
15. García-Porrúa C, González-Louzao C, Llorca J, González-Gay MA. Predictive factors for renal sequelae in adults with Henoch-Schönlein purpura. J Rheumatol. 2001; 28:1019–1024.
16. Uthman I, Kassak K, Nasr FW. Henoch-Schönlein purpura in adulthood and childhood: comment on the article by Blanco et al. Arthritis Rheum. 1998; 41:1518–1520.
17. Coppo R, Andrulli S, Amore A, Gianoglio B, Conti G, Peruzzi L, Locatelli F, Cagnoli L. Predictors of outcome in Henoch-Schönlein nephritis in children and adults. Am J Kidney Dis. 2006; 47:993–1003.
18. Pillebout E, Thervet E, Hill G, Alberti C, Vanhille P, Nochy D. Henoch-Schönlein Purpura in adults: outcome and prognostic factors. J Am Soc Nephrol. 2002; 13:1271–1278.
19. Shin JI, Park JM, Shin YH, Hwang DH, Kim JH, Lee JS. Predictive factors for nephritis, relapse, and significant proteinuria in childhood Henoch-Schönlein purpura. Scand J Rheumatol. 2006; 35:56–60.
20. Veraldi S, Mancuso R, Rizzitelli G, Gianotti R, Ferrante P. Henoch-Schönlein syndrome associated with human Parvovirus B19 primary infection. Eur J Dermatol. 1999; 9:232–233.
21. Saurina A, Botey A, Solé M, Vera M, Pou M, Torras A, Darnell A. Henoch-Schönlein purpura nephritis associated with coagulase-negative staphylococci sepsis in a patient with myeloma. Nephrol Dial Transplant. 2001; 16:2441–2442.
22. Galaria NA, Lopressti NP, Magro CM. Henoch-Schönlein purpura secondary to subacute bacterial endocarditis. Cutis. 2002; 69:269–273.
23. Pertuiset E, Lioté F, Launay-Russ E, Kemiche F, Cerf-Payrastre I, Chesneau AM. Adult Henoch-Schönlein purpura associated with malignancy. Semin Arthritis Rheum. 2000; 29:360–367.
24. Zickerman AM, Allen AC, Talwar V, Olczak SA, Brownlee A, Holland M, Furness PN, Brunskill NJ, Feehally J. IgA myeloma presenting as Henoch-Schönlein purpura with nephritis. Am J Kidney Dis. 2000; 36:E19.
25. Mitsui H, Shibagaki N, Kawamura T, Matsue H, Shimada S. A clinical study of Henoch-Schönlein Purpura associated with malignancy. J Eur Acad Dermatol Venereol. 2009; 23:394–401.
26. Borrás-Blasco J, Enriquez R, Amoros F, Cabezuelo JB, Navarro-Ruiz A, Pérez M, Fernández J. Henoch-Schönlein purpura associated with clarithromycin: case report and review of literature. Int J Clin Pharmacol Ther. 2003; 41:213–216.
27. Rauta V, Törnroth T, Grönhagen-Riska C. Henoch-Schönlein nephritis in adults-clinical features and outcomes in Finnish patients. Clin Nephrol. 2002; 58:1–8.
28. Goldstein AR, White RH, Akuse R, Chantler C. Long-term follow-up of childhood Henoch-Schönlein nephritis. Lancet. 1992; 339:280–282.
29. Schärer K, Krmar R, Querfeld U, Ruder H, Waldherr R, Schaefer F. Clinical outcome of Schönlein-Henoch purpura nephritis in children. Pediatr Nephrol. 1999; 13:816–823.
30. Kwon EH, Kim SJ, Na MA, Jung YS, Lee DW, Lee SB, Kwak IS. A clinicopathological study of the adult Henoch-Schönlein purpura. Korean J Med. 2003; 65:323–334.
31. Kim NH, Ham YR, Yoon JH, Jung JY, Kim ES, Chung S, Choi DE, Na KR, Lee KW, Shin YT. Henoch-Schönlein nephritis in adults: renal outcomes and prognostic factors. Korean J Nephrol. 2009; 28:570–578.
32. Kang Y, Ha YJ, Lee KH, Jung SY, Lee SW, Lee SK, Park YB. Clinical manifestations of Korean adult patients with Henoch-Schönlein purpura. J Korean Rheum Assoc. 2010; 17:133–142.
33. Bae CB, Lee JW, Kim HA, Jung JY, Kim HG, Lee MY, Ahn SJ, Park HL, Lee HJ, Kang E, et al. Initial hematochezia and kidney involvement are important prognostic factors of adult onset Henoch-Schönlein purpura in Korea. J Rheum Dis. 2012; 19:254–261.
34. Kim KE, Shin YH, Shin JI, Park JM, Lee JS, Jeong HJ. Clinical comparison of Henoch-Schönlein purpura nephritis in children and adults. J Korean Soc Pediatr Nephrol. 2003; 7:157–165.
35. Hong JH, Na HJ, Namgoong MK, Choi SO, Han BG, Jung SH, Kim HM. Different clinical courses of Henoch-Schönlein purpura in children, adolescents and adults. Korean J Pediatr. 2005; 48:1244–1251.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download