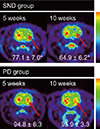
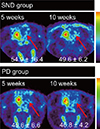
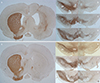
1. Fernagut PO, Tison F. Animal models of multiple system atrophy. Neuroscience. 2012; 211:77–82.
2. Kaindlstorfer C, Garcia J, Winkler C, Wenning GK, Nikkhah G, Döbrössy MD. Behavioral and histological analysis of a partial double-lesion model of parkinson-variant multiple system atrophy. J Neurosci Res. 2012; 90:1284–1295.
3. Stefanova N, Bücke P, Duerr S, Wenning GK. Multiple system atrophy: an update. Lancet Neurol. 2009; 8:1172–1178.
4. Adams RD, Vanbogaert L, Vandereecken H. Striato-nigral degeneration. J Neuropathol Exp Neurol. 1964; 23:584–608.
5. Churchyard A, Donnan GA, Hughes A, Howells DW, Woodhouse D, Wong JY, Kalnins RM, Mendelsohn FA, Paxinos G. Dopa resistance in multiple-system atrophy: loss of postsynaptic D2 receptors. Ann Neurol. 1993; 34:219–226.
6. Fernagut PO, Ghorayeb I, Diguet E, Tison F. In vivo models of multiple system atrophy. Mov Disord. 2005; 20:S57–S63.
7. Stefanova N, Tison F, Reindl M, Poewe W, Wenning GK. Animal models of multiple system atrophy. Trends Neurosci. 2005; 28:501–506.
8. Wenning GK, Granata R, Laboyrie PM, Quinn NP, Jenner P, Marsden CD. Reversal of behavioural abnormalities by fetal allografts in a novel rat model of striatonigral degeneration. Mov Disord. 1996; 11:522–532.
9. Buisson A, Pateau V, Plotkine M, Boulu RG. Nigrostriatal pathway modulates striatum vulnerability to quinolinic acid. Neurosci Lett. 1991; 131:257–259.
10. Ghorayeb I, Puschban Z, Fernagut PO, Scherfler C, Rouland R, Wenning GK, Tison F. Simultaneous intrastriatal 6-hydroxydopamine and quinolinic acid injection: a model of early-stage striatonigral degeneration. Exp Neurol. 2001; 167:133–147.
11. Venero JL, Romero-Ramos M, Revuelta M, Machado A, Cano J. Intrastriatal quinolinic acid injections protect against 6-hydroxydopamine-induced lesions of the dopaminergic nigrostriatal system. Brain Res. 1995; 672:153–158.
12. Yoon HH, Lee CS, Hong SH, Min J, Kim YH, Hwang O, Jeon SR. Evaluation of a multiple system atrophy model in rats using multitracer microPET. Acta Neurochir (Wien). 2012; 154:935–940.
13. Paillé V, Henry V, Lescaudron L, Brachet P, Damier P. Rat model of Parkinson's disease with bilateral motor abnormalities, reversible with levodopa, and dyskinesias. Mov Disord. 2007; 22:533–539.
14. Puschban Z, Scherfler C, Granata R, Laboyrie P, Quinn NP, Jenner P, Poewe W, Wenning GK. Autoradiographic study of striatal dopamine re-uptake sites and dopamine D1 and D2 receptors in a 6-hydroxydopamine and quinolinic acid double-lesion rat model of striatonigral degeneration (multiple system atrophy) and effects of embryonic ventral mesencephalic, striatal or co-grafts. Neuroscience. 2000; 95:377–388.
15. Kim JS, Lee JS, Im KC, Kim SJ, Kim SY, Lee DS, Moon DH. Performance measurement of the microPET focus 120 scanner. J Nucl Med. 2007; 48:1527–1535.
16. Hwang O, Baker H, Gross S, Joh TH. Localization of GTP cyclohydrolase in monoaminergic but not nitric oxide-producing cells. Synapse. 1998; 28:140–153.
17. Schober A. Classic toxin-induced animal models of Parkinsons disease: 6-OHDA and MPTP. Cell Tissue Res. 2004; 318:215–224.
18. Alexi T, Venero JL, Hefti F. Protective effects of neurotrophin-4/5 and transforming growth factor-alpha on striatal neuronal phenotypic degeneration after excitotoxic lesioning with quinolinic acid. Neuroscience. 1997; 78:73–86.
19. Moresco RM, Lavazza T, Belloli S, Lecchi M, Pezzola A, Todde S, Matarrese M, Carpinelli A, Turolla E, Zimarino V, et al. Quinolinic acid induced neurodegeneration in the striatum: a combined in vivo and in vitro analysis of receptor changes and microglia activation. Eur J Nucl Med Mol Imaging. 2008; 35:704–715.
20. Buisson A, Callebert J, Mathieu E, Plotkine M, Boulu RG. Striatal protection induced by lesioning the substantia nigra of rats subjected to focal ischemia. J Neurochem. 1992; 59:1153–1157.
21. Rahman A, Ting K, Cullen KM, Braidy N, Brew BJ, Guillemin GJ. The excitotoxin quinolinic acid induces tau phosphorylation in human neurons. PLoS One. 2009; 4:e6344.
22. Scherfler C, Puschban Z, Ghorayeb I, Goebel GP, Tison F, Jellinger K, Poewe W, Wenning GK. Complex motor disturbances in a sequential double lesion rat model of striatonigral degeneration (multiple system atrophy). Neuroscience. 2000; 99:43–54.
23. Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995; 15:3863–3875.
24. Köllensperger M, Stefanova N, Pallua A, Puschban Z, Dechant G, Hainzer M, Reindl M, Poewe W, Nikkhah G, Wenning GK. Striatal transplantation in a rodent model of multiple system atrophy: effects on L-Dopa response. J Neurosci Res. 2009; 87:1679–1685.
25. Köllensperger M, Stefanova N, Reindl M, Poewe W, Wenning GK. Loss of dopaminergic responsiveness in a double lesion rat model of the Parkinson variant of multiple system atrophy. Mov Disord. 2007; 22:353–358.
26. Shear DA, Dong J, Gundy CD, Haik-Creguer KL, Dunbar GL. Comparison of intrastriatal injections of quinolinic acid and 3-nitropropionic acid for use in animal models of Huntington's disease. Prog Neuropsychopharmacol Biol Psychiatry. 1998; 22:1217–1240.
27. Battisti JJ, Uretsky NJ, Wallace LJ. Sensitization of apomorphine-induced stereotyped behavior in mice is context dependent. Psychopharmacology (Berl). 1999; 146:42–48.
28. Ramaswamy S, McBride JL, Kordower JH. Animal models of Huntington's disease. ILAR J. 2007; 48:356–373.