This article has been
cited by other articles in ScienceCentral.
Abstract
Methimazole (MMI)-induced acute pancreatitis is very rare but severe adverse reaction. A 51-yr-old male developed a high fever, chills, and abdominal pain, two weeks after commencement on MMI for the treatment of Graves' disease. There was no evidence of agranulocytosis, and fever subsided soon after stopping MMI treatment. However, 5 hr after taking an additional dose of MMI, abdominal pain and fever developed again. His symptoms, biochemical, and imaging studies were compatible with acute pancreatitis. After withdrawal of MMI, he showed clinical improvement. This is the first case of MMI-induced acute pancreatitis in Korea. Clinicians should be aware of the rare but possible MMI-induced pancreatitis in patients complaining of fever and abdominal pain.
Keywords: Methimazole, Graves Disease, Pancreatitis, Drug-Related Side Effects and Adverse Reactions
INTRODUCTION
Methimazole (1-methyl-2-mercaptoimidazole, MMI) is an effective and generally safe antithyroid drug for the treatment of Graves' disease. It has been associated with various adverse reactions, which are generally minor and include skin reactions (usually urticaria or macular rashes), arthralgia, and gastrointestinal upset. Major side effects such as agranulocytosis, hepatitis, and vasculitis occur rarely, but are sometimes fatal. MMI-induced acute pancreatitis is also a major side effect (
1). Only four cases of acute pancreatitis after MMI treatment have been reported worldwide (
2,
3,
4) since the first report in 1999 (
3). In this report, we describe the clinical course, imaging and HLA typing of a case of acute pancreatitis induced by MMI in a 51-yr-old Korean male with Graves' disease and review relevant literature.
CASE DESCRIPTION
A 51-yr-old Korean male visited the emergency room with complaints of high fever, chills, and abdominal pain in August 2013. He described the pain as increasing progressively in intensity, worsening after meals, and accompanied by nausea. He also suffered from diarrhea twice. He was diagnosed with Graves' disease 2 weeks ago, and was being treated with MMI (20 mg per day). His other medications included bisoprolol and 100 mg aspirin for atrial fibrillation. He did not have any history of drug or food allergies. He denied the use of tobacco, alcohol, and any dietary supplements. His family history was unremarkable, and did not include thyroid disease or gastrointestinal disorders.
Upon admission, his vital signs were as follows: blood pressure of 100/70 mmHg, heart rate of 121 beats per minute, a respiratory rate of 21 times per minute, and a body temperature of 39.0℃. He had a slightly enlarged thyroid gland, but there was no pharyngeal injection. Physical examination revealed tenderness in the epigastric and upper abdomen without rebound tenderness, guarding, or rigidity.
The results of blood tests were as follows: white blood cell count of 5,460/µL, hemoglobin of 16.3 g/dL, and platelet count of 103,000/µL. Biochemical parameters were glucose of 222 mg/dL, triglycerides of 113 mg/dL, blood urea nitrogen of 30.3 mg/dL, creatinine of 1.45 mg/dL, sodium of 133.8 mM/L, potassium of 3.9 mM/L, chloride of 97.3 mM/L, aspartate transaminase of 34 IU/L, alanine transaminase of 46 IU/L, alkaline phosphatase of 46 U/L, total bilirubin of 2.60 mg/dL, C-reactive protein (CRP) of 46.7 mg/L, amylase of 86 IU/L (normal range: 29-110), and lipase of 86 IU/L (normal range: 14-60).
A thyroid function test at the first visit revealed TSH of 0.01 mIU/L (normal range: 0.27-4.20), free T4 of 3.89 ng/dL (normal range: 0.93-1.70), total T3 of 199.8 ng/dL (normal range: 80-200), anti-TPO antibody of 17.84 (normal range: 0-34), and TSH-receptor antibody of 8.8 IU/L (normal range: 0.00-1.75). In Tc-99m thyroid scan, the uptake ratio was 4.8%. There was no elevation of serum antibodies for respiratory viruses including adenovirus, influenza, parainfluenza, and rhinovirus. He also tested negative for serum antibodies against tsutsugamushi, leptospira, and hantavirus.
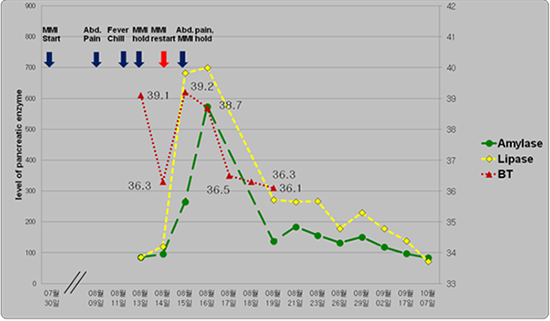
The following day, MMI therapy was restarted after holding 42 hr because his fever had subsided and there was no evidence of agranulocytosis. However, 5 hr after he took the second dose of MMI (10 mg), abdominal pain and fever (39.2℃) developed again. At that time, laboratory studies showed a white blood cell count of 5,580/µL (72.7% neutrophils and 1.9% eosinophils), total bilirubin of 1.84 mg/dL, and CRP of 26.0 mg/L. His serum amylase and lipase levels increased to 265 IU/L and 682 IU/L, respectively. Electrophoresis revealed that his pancreatic amylase isoenzyme levels were elevated. An abdominal CT revealed a swollen pancreas with peripancreatic inflammatory fat stranding, suggestive of acute pancreatitis (
Fig. 1A). An abdominal ultrasound was then performed, which showed no evidence of cholelithiasis or biliary duct dilatation (
Fig. 1B).
After the withdrawal of MMI, his fever and abdominal pain improved, and pancreatic enzyme levels began to decrease and finally normalized (
Fig. 2). We measured IgG and IgG4 to exclude autoimmune pancreatitis. The serum levels of IgG and IgG subclass-4 were in the normal range (901.5 mg/dL, normal range: 700-1,600; and 349 mg/L, normal range: 30-2,010, respectively). The serologic typing of HLA alleles revealed DRB1
*0803 and DQB1
*0601. After discharge, PTU (50 mg t.i.d) was prescribed for treatment of Graves' disease and his hyperthyroidism was gradually controlled without any side-effects.
DISCUSSION
Acute pancreatitis is recently recognized as a rare major adverse event of MMI. There have been only four reports of MMI-induced acute pancreatitis since the first report in 1999 (
2,
3,
4).
Because of the absence of cause-specific tests, the diagnosis of drug-induced pancreatitis (DIP) is usually based on the following criteria (
5): 1) acute pancreatitis developed during the administration of a drug; 2) resolution of symptoms upon withdrawal of the drug; 3) recurrence after a rechallenge with the suspected agent; and 4) exclusion of all other common causes. In this case report, acute pancreatitis occurred 14 days after starting MMI in a 51-yr-old male. The episodic fever and abdominal pain subsided shortly after the withdrawal of the drugs. When MMI was re-administered, fever and abdominal pain rapidly reappeared. DIP by MMI was confirmed because there was no evidence of cholelithiasis, pancreatic and bile duct dilatation, or history of alcohol use to cause pancreatitis, normal triglyceride level, and the recurrence of pancreatitis occurred after the unintended rechallenge with MMI.
The diagnosis of MMI-induced DIP is commonly overlooked compared to drug-induced liver disease for several reasons (
6). First, the index of suspicion for DIP is considerably lower than drug-induced hepatotoxicity. Unlike transaminases, serum amylase and lipase levels are not part of the metabolic profile obtained during a routine health checkup because of the differences in the latency period between the time of exposure and the development of acute pancreatitis; therefore, it takes an astute and motivated physician to diagnose DIP. In addition, even when DIP is diagnosed it is reported only rarely, and many cases might be often erroneously classified as being alcoholic or biliary in etiology by default. Finally, treatment invariably includes making the patient nil-per-os (NPO), which results in the inadvertent discontinuation of the possibly offending medication, which can remove the opportunity to diagnose DIP.
Although cases of acute pancreatitis after MMI with positive rechallenge have been reported previously (
3), detection of this adverse event has not been emphasized due to its low incidence. However, the accumulation of reports of such cases will encourage clinicians to suspect MMI-induced acute pancreatitis and to discontinue the use of MMI when patients taking MMI complain of abdominal pain and fever. Therefore, more active and systematic efforts will be necessary to detect cases and understand the mechanism by which MMI induces a hypersensitivity reaction or injures the pancreas.
Four of the five cases of MMI-induced DIP were Asian and one was Caucasian, and all were females, and thus this is the first report in a male subject. We initially suspected that genetic susceptibility or an autoimmune mechanism could be involved in MMI-induced pancreatitis in our patient. Therefore, we analyzed the patient's HLA haplotype to identify the type frequently found in Korean Graves' disease and confirm that it was congruous with that of autoimmune pancreatitis (AIP). The HLA DRB1
*0405-DQB1
*0401 haplotype was associated with AIP in a Japanese study (
7), but this did not correspond with the haplotype of our patient. However, the HLA DRB1
*0803 and DQB1
*0601 detected in this patient have been linked with autoimmune thyroid diseases such as Graves' disease, and have been reported to play a protective role in Type 1 diabetes (
8). Type 1 AIP is more common in Asian subjects and in males, and is accompanied by elevated serum IgG, IgG4, and positive autoantibodies (
9). IgG4 plays a major role in the pathogenesis of AIP (
10), but IgG4 levels were not increased in our patient. We also excluded autoimmune type 1 diabetes because of negative anti-insulin and anti-GAD antibodies, which suggested that his hyperglycemia could have been caused by thyrotoxicosis.
In summary, we describe a 51-yr-old Korean male who developed acute pancreatitis after the administration of MMI for Graves' disease. He presented with acute pancreatitis after taking MMI for 14 days, and his fever and abdominal pain subsided after the withdrawal of MMI. When MMI was re-administered, acute pancreatitis rapidly recurred, which was confirmed by clinical, biochemical, and imaging studies. Acute pancreatitis is a rare but severe adverse reaction induced by MMI. If a patient taking MMI complains of abdominal symptoms and fever without neutropenia, clinicians should consider the rare possibility of MMI-induced pancreatitis and exclude all other potential causes of acute pancreatitis during diagnosis. Even after the pancreatitis has improved, MMI should not be administered again.














 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download