Abstract
Acute kidney injury (AKI) is closely associated with the mortality of hospitalized patients and long-term development of chronic kidney disease, especially in children. The purpose of our study was to assess the evidence of contrast-induced AKI after cardiac catheterization in children with heart disease and evaluate the clinical usefulness of candidate biomarkers in AKI. A total of 26 children undergoing cardiac catheterization due to various heart diseases were selected and urine and blood samples were taken at 0 hr, 6 hr, 24 hr, and 48 hr after cardiac catheterization. Until 48 hr after cardiac catheterization, there was no significant increase in serum creatinine level in all patients. Unlike urine kidney injury molecule-1, IL-18 and neutrophil gelatinase-associated lipocalin, urine liver-type fatty acid-binding protein (L-FABP) level showed biphasic pattern and the significant difference in the levels of urine L-FABP between 24 and 48 hr. We suggest that urine L-FABP can be one of the useful biomarkers to detect subclinical AKI developed by the contrast before cardiac surgery.
Acute kidney injury (AKI) is closely associated with mortality of hospitalized patients and long-term development of chronic kidney disease (1). In pediatric patients, AKI is also regarded as an important factor to decide long-term outcome. There are two clinical guidelines for the definition of AKI in children; pRIFLE (the pediatric Risk, Injury, Failure, Loss, End-Stage Kidney Disease) criteria and AKIN (the Acute Kidney Injury Network) staging (2, 3). Although serum creatinine was used as a biomarker to evaluate renal function in these guidelines, it has been reported that serum creatinine is not adequate for the prediction of precise outcome and the evidence of prophylactic treatment because the level of serum creatinine usually increases only after marked decrease of glomerular filtration rate (GFR) (1). Moreover, the level of serum creatinine can vary due to various extra-renal factors such as the amount of muscle, diet and drugs.
Recently, many novel biomarkers in AKI, rather than serum creatinine as a classical marker of GFR, have been developed to directly detect tubular injury, as well as facilitate early intervention. Candidate biomarkers in AKI include neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), interleukin-18 (IL-18) and liver-type fatty acid-binding protein (L-FABP) (1).
In actual clinical circumstances, cardiopulmonary bypass for open heart surgery is well known as an AKI-inducible condition, in particular subclinical AKI that cannot be detected by serum creatinine. In addition, contrast-induced AKI, previously known as contrast-induced nephropathy (CIN) is also another important AKI-inducible condition (4, 5, 6).
The purpose of our study is to assess the evidence of contrast-induced AKI after cardiac catheterization in children with heart disease and evaluate the clinical usefulness of NGAL, KIM-1, IL-18, and L-FABP as the candidate biomarkers in AKI.
A total of 26 children undergoing cardiac catheterization was selected from January 2009 to February 2011 at Kyungpook National University Hospital. All patients had various heart diseases such as: ventricular septal defect, atrial septal defect, patent ductus arteriosus, tetralogy of Fallot, and pulmonary atresia. In 26 children, only one patient with pulmonary atresia showed cyanosis. Mean fractional shortening on echocardiogram was 37.8% (31.4%-49.4%) and none had symptoms and signs of heart failure. In addition, none had underlying renal diseases. The mean age of patients was 7.1 yr, and the male to female ratio was 12:14. The children were admitted to the hospital one day before cardiac catheterization. In 15 of the 26 children, urine and blood samples were taken at 0 hr (before catheterization), then 6 hr and 24 hr after the procedure; the change of KIM-1 level of the urine samples were analyzed. In the other 11 of 26 children, urine and blood samples were taken at 0 hr, 6 hr, 24 hr, and 48 hr after cardiac catheterization, and the change of IL-18, L-FABP and NGAL level as well as KIM-1 of the urine samples were analyzed (Table 1). All urine samples were collected via urine bags (only in young infants) or taken during mid-stream by the time appointed.
The process of cardiac catheterization was as follows: each patient's femoral artery and vein were punctured, and the contrast was injected with a speed of 10 mL per second. After the injection, we observed the vessels and each chamber of heart by fluoroscopy. The contrast used was Ultravist®, produced by Bayer Healthcare and composed of iopromide; it is a low osmolar, non-ionic contrast agent for intravascular use. The average contrast dose was 2.5 mL per kilogram.
Urine samples were collected using a sterile container. Particulates were removed by centrifugation for 15 min at 1,000 g, and the samples stored at -80℃ until use.
The ELISA was performed according to the manufacturer's instructions. Briefly, microtiter plates pre-coated with a monoclonal antibody against human KIM-1 (#CSB-E08807H, Cusabio, China), NGAL (#KIT036, Bioporto, Denmark), IL-18 (E90064Hu, USCN Life Science, Houston, TX, USA) or L-FABP (E91566Hu, USCN Life Science) were added with 100 µL of urine samples or standards for 1 hr at 37℃. After removed the liquid, each well was incubated with a 100 µL biotinylated monoclonal antibody for 1 hr at 37℃. The solution was aspirated and washed 3 times, followed by 100 µL avidin-conjugated HRP treatments for 1 hr at 37℃. After aspirate and washing 5 times, 100 µL tetramethylbenzidine substrate was added for color development in the dark room, which was read after 10-30 min at 450 nm with ELISA reader (Benchmark Plus, BioRad, Hercules, CA, USA). All measurements were made in triplicate.
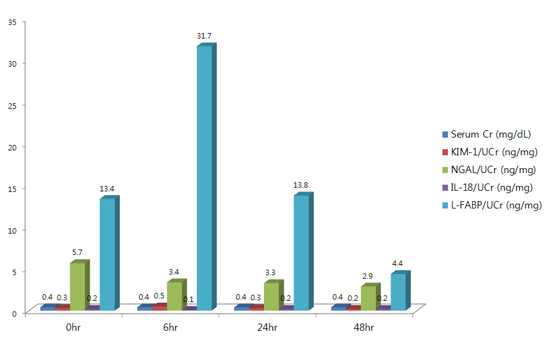
The mean levels of serum creatinine (mg/dL) at 0 hr, 6 hr, 24 hr, and 48 hr after cardiac catheterization were 0.4, 0.41, 0.41, and 0.40, respectively. Until 48 hr after cardiac catheterization, there was no significant increase in serum creatinine level in all patients.
Urine KIM-1 levels showed a tendency to increase as time elapsed until 48 hr after the cardiac catheterization. The level of urine KIM-1 at 48 hr (133.2±136.9 pg/mL) after procedure was significantly higher than that at 0 hr (70.9±80.8 pg/mL) and 6 hr (78.3±52.5 pg/mL) after procedure (0 vs. 48 hr, P=0.017; 6 vs. 48 hr, P=0.011) (Table 2 and Fig. 1). However, the pattern of the results was changed when urine KIM-1 was expressed as ng/mg creatinine. The level of urine KIM-1 (ng/mg creatinine) at 6 hr was higher than that at other times, but did not show the statistical significance.
Urine NGAL levels also showed a tendency to increase as time elapsed until 48 hr after the cardiac catheterization. However, because urine NGAL level at 6 hr after the procedure decreased to the baseline level (0 hr) of urine NGAL and there was no significant difference in the level of NGAL between 0 hr and 24 or 48 hr after the procedure, we regarded that there was no significant change with time in urine NGAL level; likewise urine IL-18 did not show any significant changes (Table 2).
However, urine L-FABP level showed biphasic patterns, increasing at 6 hr (9,599.4±7,771.6 pg/mL) after the procedure compared to the baseline level (0 hr, 5,276.6±6,474.6 pg/mL) and then the decreasing until 48 hr (24 and 48 hr after the procedure). There was a significant difference in the levels of urine L-FABP between 24 hr (8,953.6±9,577.8 pg/mL) and 48 hr (4,480.6±8,326.2 pg/mL) (P=0.019) (Table 2, Fig. 2). When the values of urine IL-18, NGAL and L-FABP except for urine KIM-1 were adjusted using urine creatinine (from pg/mL to ng/mg creatinine), the pattern of results was unchanged.
CIN is a complex syndrome of acute renal failure developed after the infusion of iodinated contrast media. There are various risk factors of CIN including intra-arterial administration, ionic high-osmolality agents, chronic kidney disease and diabetes mellitus (4). In particular, the osmolality of contrast medium seems to play an important role in the development of CIN. Thus, to prevent AKI, low-osmolar contrast agents are more desirable than high-osmolar agents due to rapid iodine clearance (4, 7). The pathophysiologic mechanism of CIN is poorly understood. However, it has been suggested that the development of CIN is associated with the vasoconstriction effects on renal blood flow, which may be triggered by an influx of calcium ion, release of endothelin, impaired nitric oxide production, as well as direct tubular toxicity by contrast medium. And these pathophysiologic mechanisms tend to be more intensely and persistently caused by ionic high-osmolality agents (4). The most common definition of CIN is a relative rise in serum creatinine >25% from the baseline value or an absolute increase >0.5 mg/dL within 48 hr after the administration of contrast medium (5). In this study, there was no such significant elevation of serum creatinine to meet that criterion of definite CIN. This result suggest that a low osmolar, non-ionic contrast agent for intravascular use such as Ultravist® seldom produces the definite AKI, thus we need more sensitive biomarkers to detect subclinical AKI developed from the contrast than serum creatinine.
Recently, it was reported that renal tubular damage without the rise of serum creatinine is also associated with a worse renal outcome, this condition is called as 'subclinical AKI' (8). Ronco et al. suggested new conceptual framework for contrast-induced AKI (CI-AKI) based on the functional criteria such as RIFLE and AKIN, and structural criteria such as novel biomarkers including KIM-1, IL-18, NGAL, and L-FABP (6). For example, if functional criteria are not met, but structural criteria are met, this condition would be described as CI-AKI with only structural damage and compatible with subclinical AKI. If functional criteria are met, but structural criteria are not met, this condition would be described as CI-AKI with only renal dysfunction (6).
Cardiac surgery using cardio-pulmonary bypass has been regarded as most useful clinical situation of the induction to AKI (9, 10), so the detection of AKI is very important for patients with congenital heart diseases who need open heart surgery in the future. Because cardiac catheterization using contrast is essential for the accurate diagnosis with congenital heart disease before cardiac surgery, the early detection of subclinical AKI is more important in these patients than other clinical situations, in order to prevent clinical AKI (11).
NGAL is a 25 kDa-lipocalin secreted from the activated neutrophils, and increases when there are conditions such as: infection, malignancy and renal tubular injury. In the kidney, NGAL is usually expressed in the Henle loop and distal tubules and can be expressed in the proximal tubules reabsorbed after glomerular filtration (13, 14). For children underwent a cardiopulmonary bypass, it has been reported that patients with prominent elevations of NGAL in serum and urine over 2 hr after operation tend to develop AKI later (15). Hirsch et al. reported that elevation of NGAL in serum and urine of children who underwent a cardiac catheterization using contrast medium can be the predictive factors to CI-AKI (16). However, in this study, there was no significant elevation of urine NGAL levels after cardiac catheterization. We speculate that this difference originated from the lack of patients presenting the definite CIN, because we used a low-osmolar, non-ionic contrast agent, less amount of contrast, and had a smaller numbers of patients with cyanotic heart diseases, compared to the previous report (16). Therefore, we suggest that urine NGAL has some limitations to diagnose the subclinical AKI developed from use of contrast in children.
KIM-1, type I transmembrane glycoprotein of 104 kDa, is little expressed in a normal kidney, whereas it increases prominently in the proximal tubules after ischemic or toxic renal injury (1). If renal tubular injury develops, the extracellular part of KIM-1 is separated by proteolytic enzymes and secreted in the urine (17, 18). In critically ill patients with various types of acute tubular necrosis, elevation of urine KIM-1 is closely related to the increase of the mortality, and necessitates renal replacement therapies (19). Han et al. also reported that urine KIM-1 after 12 hr of cardiac surgery is a predictive factor of AKI in children (20). In this study, urine KIM-1level presented a tendency to increase in progress with time after injection of the contrast. The level of urine KIM-1 at 48 hr after the procedure was the highest and significantly higher than that at 0 and 6 hr after the procedure. However, the pattern of the results was changed when urine KIM-1 was expressed as ng/mg creatinine. The level of urine KIM-1 (ng/mg creatinine) at 6 hr was higher than that at other times, but did not show the statistical significance. Therefore, we suggest that clinical usefulness of urine KIM-1 should be confirmed by further larger, controlled trials.
IL-18, an 18 kDa pro-inflammatory cytokine, is activated by caspase-1 and found in the tubular epithelial cells during AKI (21). In animal models, it was reported that neutralizing antibody of IL-18 prevents ischemic renal injury, thus IL-18 appears to play an important role on the development of ischemic renal injury (22). Parikh et al. suggested that the change of serum creatinine after renal transplantation can be anticipated based on the concentration of urine IL-18 on the day of the renal transplantation (23). We suggest that urine IL-18 would not be a meaningful candidate for a contrast-induce AKI biomarker, because in this study urine IL-18 did not show any significant change due to the contrast.
L-FABP, a 14 kDa protein, is expressed in the proximal tubules and related to the metabolism of urine free fatty acid. FABPs as intracellular carrier proteins are classified into nine types according to the distributed organs (24). Wang et al. (25) reported that L-FABP has the effect on the reduction of oxidative stress originated from ischemic reperfusion injury in an in vitro study. In animal studies of ischemic or toxic renal injury, L-FABP increases within several minutes or hours after renal injury, and correlates well with the degree of pathologic injury, thus L-FABP is a valuable biomarker for the early detection of AKI (26, 27). Portilla et al. (28) also reported that the elevation of urine L-FABP 4 and 12 hr after cardiac surgery in 40 children is significantly correlated with the predicted AKI. In addition, it was reported that urine L-FABP is an important biomarker of transient renal tubular injury in CI-AKI (29). In this study, urine L-FABP level showed biphasic patterns, increasing at 6 hr after the procedure compared to the baseline level (0 hr) and then decreasing until 48 hr (24 and 48 hr after the procedure). There was a significant difference in the levels of urine L-FABP between 24 hr and 48 hr. Therefore, we suggest that urine L-FABP can be a useful, early biomarker of AKI developed by the contrast in children.
By the way, in the previous reports of novel AKI biomarkers, we can easily find the differences of results according to various clinical situations including cardiac surgery, contrast nephropathy, sepsis and kidney transplant (9). For example, in patients undergoing cardiac surgery, NGAL was an early AKI biomarker and IL-18 was an intermediate AKI biomarker whereas, in contrast nephropathy, IL-18 was not a significant AKI biomarker like our result. Although the precise mechanism about the differences in the changes of each biomarker according to clinical situations is poorly revealed yet, it has been suggested that the specific AKI biomarker according to various clinical situations should be confirmed to optimize the early detection and intervention of subclinical AKI.
In summary, urine L-FABP levels showed the significant elevation after cardiac catheterization, whereas urine NGAL and IL-18 did not show any significant change in the levels after the procedure and urine KIM-1 did not present the consistent results according to adjustment of urine creatinine. Therefore, we suggest that urine L-FABP can be one of the useful biomarkers to detect subclinical AKI caused by contrast before cardiac surgery.
Figures and Tables
Fig. 1
Change of urine KIM-1 at time after cardiac catheterization using contrast. KIM-1, kidney injury molecule-1. 0 vs. 48 hr, P = 0.017; 6 vs. 48 hr, P = 0.011.

Fig. 2
Change of urine L-FABP at time after cardiac catheterization using contrast. L-FABP, liver-type fatty acid-binding protein. 24 vs. 48 hr, P = 0.019.

Notes
References
1. Al-Ismaili Z, Palijan A, Zappitelli M. Biomarkers of acute kidney injury in children: discovery, evaluation, and clinical application. Pediatr Nephrol. 2011; 26:29–40.
2. Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007; 71:1028–1035.
3. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007; 11:R31.
4. Tumlin J, Stacul F, Adam A, Becker CR, Davidson C, Lameire N, McCullough PA. CIN Consensus Working Panel. Pathophysiology of contrast-induced nephropathy. Am J Cardiol. 2006; 98:14K–20K.
5. Finn WF. The clinical and renal consequences of contrast-induced nephropathy. Nephrol Dial Transplant. 2006; 21:i2–i10.
6. Ronco C, Stacul F, McCullough PA. Subclinical acute kidney injury (AKI) due to iodine-based contrast media. Eur Radiol. 2013; 23:319–323.
7. Lenhard DC, Pietsch H, Sieber MA, Ernst R, Lengsfeld P, Ellinghaus P, Jost G. The osmolality of nonionic, iodinated contrast agents as an important factor for renal safety. Invest Radiol. 2012; 47:503–510.
8. Ronco C, Kellum JA, Haase M. Subclinical AKI is still AKI. Crit Care. 2012; 16:313.
9. Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008; 3:665–673.
10. Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006; 1:19–32.
11. Bianchi P, Carboni G, Pesce G, Isgrò G, Carlucci C, Frigiola A, Giamberti A, Ranucci M. Cardiac catheterization and postoperative acute kidney failure in congenital heart pediatric patients. Anesth Analg. 2013; 117:455–461.
12. Devarajan P. Biomarkers for the early detection of acute kidney injury. Curr Opin Pediatr. 2011; 23:194–200.
13. Devarajan P. Neutrophil gelatinase-associated lipocalin: an emerging troponin for kidney injury. Nephrol Dial Transplant. 2008; 23:3737–3743.
14. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005; 115:610–621.
15. Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005; 365:1231–1238.
16. Hirsch R, Dent C, Pfriem H, Allen J, Beekman RH 3rd, Ma Q, Dastrala S, Bennett M, Mitsnefes M, Devarajan P. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007; 22:2089–2095.
17. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002; 62:237–244.
18. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998; 273:4135–4142.
19. Liangos O, Perianayagam MC, Vaidya VS, Han WK, Wald R, Tighiouart H, MacKinnon RW, Li L, Balakrishnan VS, Pereira BJ, et al. Urinary N-acetyl-beta-(D)-glucosaminidase activity and kidney injury molecule-1 level are associated with adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007; 18:904–912.
20. Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008; 73:863–869.
21. Leslie JA, Meldrum KK. The role of interleukin-18 in renal injury. J Surg Res. 2008; 145:170–175.
22. Melnikov VY, Faubel S, Siegmund B, Lucia MS, Ljubanovic D, Edelstein CL. Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J Clin Invest. 2002; 110:1083–1091.
23. Parikh CR, Jani A, Mishra J, Ma Q, Kelly C, Barasch J, Edelstein CL, Devarajan P. Urine NGAL and IL-18 are predictive biomarkers for delayed graft function following kidney transplantation. Am J Transplant. 2006; 6:1639–1645.
24. Chmurzyńska A. The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet. 2006; 47:39–48.
25. Wang G, Gong Y, Anderson J, Sun D, Minuk G, Roberts MS, Burczynski FJ. Antioxidative function of L-FABP in L-FABP stably transfected Chang liver cells. Hepatology. 2005; 42:871–879.
26. Nakamura T, Sugaya T, Node K, Ueda Y, Koide H. Urinary excretion of liver-type fatty acid-binding protein in contrast medium-induced nephropathy. Am J Kidney Dis. 2006; 47:439–444.
27. Negishi K, Noiri E, Doi K, Maeda-Mamiya R, Sugaya T, Portilla D, Fujita T. Monitoring of urinary L-type fatty acid-binding protein predicts histological severity of acute kidney injury. Am J Pathol. 2009; 174:1154–1159.
28. Portilla D, Dent C, Sugaya T, Nagothu KK, Kundi I, Moore P, Noiri E, Devarajan P. Liver fatty acid-binding protein as a biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2008; 73:465–472.
29. Kato K, Sato N, Yamamoto T, Iwasaki YK, Tanaka K, Mizuno K. Valuable markers for contrast-induced nephropathy in patients undergoing cardiac catheterization. Circ J. 2008; 72:1499–1505.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download