Abstract
Figures and Tables
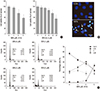 | Fig. 1WFA causes apoptosis in rabbit articular chondrocytes. (A) Articular chondrocytes were untreated or treated with the indicated concentrations of WFA for 24 hr (left panel) or with 5 µM WFA for the indicated time periods (right panel). Cell viability was determined by MTT assay. (B) Rabbit articular chondrocytes were untreated or treated with 5 µM WFA for 24 hr. Fragmented nucleus of cells was stained with 4'-6-diamidino-2-phenylindole (DAPI) using fluorescence microscope (magnification, ×400). (C) Cells were untreated or treated with the indicated concentrations of WFA for 24 hr. Flow cytometric analysis of apoptotic cells was performed using propidium iodide (PI). The data are representative result of three similar experiments. Values are means ± S.E.M. of three experiments (n = 3); statistically significant: *P < 0.01 (versus the corresponding untreated group). |
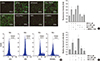 | Fig. 2WFA triggers generation of intracellular ROS. (A) Articular chondrocytes were untreated or treated with 5 µM WFA or 200 µM hydrogen peroxide (H2O2) for 1 hr in the absence or presence of 5 mM N-acetyl cysteine (NAC, an inhibitor of intracellular ROS) or 1 mM NG-monomethyl-L-arginine (L-NMMA, a nitric oxide synthase inhibitor). Hydrogen peroxide (H2O2) was used as a positive control. Chondrocytes were stained with DCFH-DA for 30 min and analyzed with fluorescence microscopy (left panel). Intensity of fluorescence was determined by image j program (right panel). (B) Chondrocytes were untreated or treated with 5 µM WFA for 1 hr in the absence or presence of 5 mM N-acetyl cysteine (NAC, an inhibitor of intracellular ROS). Cells were stained with DCFH-DA and 2,000 events analyzed in flow cytometric analysis. (C) Articular chondrocytes were untreated or treated with 5 µM WFA or 200 µM H2O2 for 1 hr in the absence or presence of 5 mM NAC. The production of intracellular ROS was evidenced by an increase in intensity of the DCF signal using the FLx8000 Bio-Tek fluorometer. The data are representative result of three similar experiments. Values are means ± SEM of three experiments (n = 3); statistically significant: *P < 0.01 (versus the corresponding untreated group). |
 | Fig. 3Treatment of NAC inhibits WFA-induced apoptosis. (A-C) Articular chondrocytes were untreated or treated with 5 µM WFA for 24 hr in the absence or presence of 5 mM NAC or 1 mM L-NMMA. (A) Cell viability was determined by MTT assay. (B) Flow cytometric analysis of apoptotic cells was performed using propidium iodide (PI). (C) Fragmented nucleus of cells was stained with 4'-6-diamidino-2-phenylindole (DAPI) using fluorescence microscope (magnification, ×400). The data are representative result of three similar experiments. Values are means ± SEM of three experiments (n = 3); statistically significant: *P < 0.01 (versus the corresponding untreated group). |
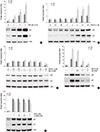 | Fig. 4WFA increases the expression of p53 and p21. (A) Chondrocytes were untreated or treated with the indicated concentrations of WFA for 24 hr. (B) Chondrocytes were untreated or treated with 5 µM WFA for the indicated time periods. (C) Cells were untreated or treated with 5 µM WFA in the absence or presence of 5 mM NAC for the specified time periods. (D) Chondrocytes were untreated or treated with 5 µM WFA in the absence or presence of the increased concentrations of NAC for 24 hr. (E) Cell were untreated or treated with 5 µM WFA in the absence or presence of the indicated concentrations of L-NMMA for 24 hr. (A-E) Expressions of p53 and p21 were analyzed by Western blot analysis. Actin was used as loading control. The data are representative result of three similar experiments. Values are means ± SEM of three experiments (n = 3); statistically significant: *P < 0.01 (versus the corresponding untreated group). |
 | Fig. 5WFA induces the phosphorylation of PI3K and JNKinase. (A) Rabbit chondrocytes were untreated or treated with the indicated concentrations of WFA for the indicated time periods. (B) Articular chondrocytes were untreated or treated with 5 µM WFA for the indicated time periods. (C) Cells were untreated or treated with 5 µM WFA in the absence or presence of 5 mM NAC for the specified time periods. (A-C) Activation of Akt, JNK and pc-Jun was analyzed by Western blot analysis. Actin was used as loading control. The data are representative result of three similar experiments. |
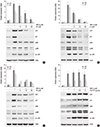 | Fig. 6Inhibition of PI3K/Akt and JNK with inhibitors, LY/TB or SP, or activator, 740 Y-P is blocked the expression of p53 and p21. (A) Chondrocytes were untreated or treated with 5 µM WFA for 24 hr in the absence or presence of the indicated concentrations of SP600125 (SP). (B) Articular chondrocytes were untreated or treated with 5 µM WFA in the absence or presence of the indicated concentrations of LY294002 (LY). (C) Chondrocytes were untreated or treated with 5 µM WFA in the absence or presence of the indicated concentrations of triciribine (TB, an inhibitor of Akt). (D) Chondrocytes were untreated or treated with 5 µM WFA in the absence or presence of the indicated concentrations of 740 Y-P (an activator of PI3K). (A-D) Expressions of p53 (24 hr), p21 (24 hr), pJNK (10 min), pc-Jun (3 hr), pAkt (6 hr), and actin (24 hr) and actin were detected by Western blot analysis. Expression of actin was used as a loading control. The data are typical results from four independent experiments with similar results. |
 | Fig. 7Blockage of PI3K/Akt and JNKinase pathways inhibits WFA-induced apoptosis. (A-D) Rabbit articular chondrocytes were untreated or treated with 5 µM WFA for 24 hr in the absence or presence of NAC, SP600125 (SP), or LY294002 (LY). (A) Cell viability was determined by MTT assay. (B) Flow cytometric analysis of apoptotic cells was performed using propidium iodide (PI). (C) Fragmented nucleus of cells was stained with 4'-6-diamidino-2-phenylindole (DAPI) using fluorescence microscope (magnification, ×400). (D) The production of intracellular ROS was evidenced by an increase in intensity of the DCF signal using the FLx8000 fluorometer. The data are representative result of three similar experiments. Values are means ± SEM of three experiments (n = 3); statistically significant: *P < 0.01 (versus the corresponding untreated group). |
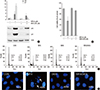 | Fig. 8Treatment of DIDS reversed WFA-caused apoptosis. (A-D) Primary chondrocytes were untreated or treated with 5 µM WFA for 24 hr in the absence or presence of 4,4'44FA for 24 hr in the absence disulfonic acid (DIDS, an mitochondrial inner membrane anion channel). (A) Expressions of p53 and p21 were analyzed by Western blot analysis. Actin was used as a loading control. (B) Cell viability was determined by MTT assay. (C) Flow cytometric analysis of apoptotic cells was performed using propidium iodide (PI). (D) Fragmented nucleus of cells was stained with 4'-6-diamidino-2-phenylindole (DAPI) using fluorescence microscope (magnification, ×400). The data are typical results from four independent experiments with similar results. Values are means ± SEM of three experiments (n = 3); statistically significant: *P < 0.01 (versus the corresponding untreated group). |
Notes
Funding: This work was supported by the Korean Health Technology R&D project, Ministry of Health & Welfare, Republic of Korea (A120960-1201-0000300) and the Korea Foundation for the Advancement of Science & Creativity (KOFAC), and funded by the Korean Government (MOE), and also by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MEST)(NRF-2012R1A1A2043276).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download