Abstract
Allergen-specific immunotherapy (SIT) reduces allergen specific IgE (sIgE) levels and achieves clinical and immunological tolerance by modulating innate and adaptive immunological responses. Increased temperature and CO2 concentrations caused by climate changes contribute to an increase of pollen count and allergenicity that influences clinical SIT outcomes. In this study, we investigated the changes of IgE binding components to tree and weed pollens in pollinosis patients who showed a paradoxical increase of serum sIgE level during pollen-SIT. We enrolled nine patients who showed an increasing pattern of serum sIgE level to alder, birch, ragweed and mugwort pollens by enzyme-linked immunosorbant assay. IgE immunoblot analysis confirmed the intensification or new generation of major IgE binding components that could be induced by climate change. The findings suggest that the regular monitoring of sIgE levels and symptom changes is required to improve the clinical outcomes of SIT in patients undergoing SIT for tree and weed pollens.
Pollen grains are the second most common allergen that cause allergic diseases (pollinosis) such as allergic rhinitis, conjunctivitis, asthma and atopic dermatitis (1, 2, 3, 4, 5, 6). Most allergenic tree pollens in Korea are Betulaceae (alder, hazelnut, and birch) that appear in early spring (late February) and the allergenic weed pollens are mostly Asteraceae (mugwort) and Ambrosiae (ragweed) that appear in autumn (1, 2). A diagnosis of pollinosis can be made based on allergic rhinitis (AR), allergic conjunctivitis (AC), bronchial asthma (BA) and atopic dermatitis (AD) with seasonal aggravating symptoms. An allergy skin test and/or the measurement of serum specific IgE level identify the offending allergens. Radioallergosorbent test (RAST), multiple allergen simultaneous test (MAST) and capsulated hydrophilic carrier polymer (CAP) system (ImmunoCAP®, ThermoFisher Scientific, Uppsala, Sweden) detect serum specific IgE (sIgE) antibodies in various allergens that include pollen (2). Allergen-specific immunotherapy (SIT) is widely used to improve allergic and systemic symptoms as well as prevent disease progression, development of new sensitization, and the onset of asthma that sustained even after the cessation of SIT (7, 8). SIT could achieve clinical and immunological tolerance by modulating innate and adaptive immunological responses that reduce allergen specific IgE (sIgE) levels (9). However, recent environmental changes (global warming and increased CO2 concentrations) have changed the concentration of major allergens in pollens such as Amb a 1 and Bet v 1 (10) and the allergenic components of major pollens (11,12) that could influence clinical outcomes. The 2012 Korean pollen calendar demonstrated that allergenic flowers blossom earlier and fall later compared to previous nationwide surveys from 1997 to 2002, which contributed to increased pollinosis over the last 10 yr (1, 11, 12, 13, 14, 15, 16). In addition, increased allergenic potency with environmental change was noted in Japanese Hop pollens presented as the intensification of the major allergenic component at 10 kDa and the development of new sensitization to additional IgE binding components (12). However, little investigations have been made in pollinosis patients who showed increased tendency of sIgE levels even though treated with allergen-SIT.
We investigated the changes of IgE binding components to major tree and weed pollens (alder, birch, ragweed, and mugwort) (1, 12) in pollinosis patients undergoing allergen-SIT. We enrolled nine pollinosis patients with an increasing tendency of serum sIgE levels or A/H (allergen/histamine wheal) ratio on following skin prick tests (SPTs) to alder, birch, ragweed and mugwort pollens during the pollen-SIT from the Allergy and Clinical Immunology Department of Ajou University Hospital, Suwon, Korea. We analyzed the clinical data (including age, sex, underlying allergic diseases, SPTs, and serum specific IgE level) and investigated the underlying allergic diseases (AR, AC, BA, and AD). The A/H ratio represents the results of SPTs performed with 50 common aeroallergens (Bencard, Bretford, UK). Serum sIgE levels to each pollen were monitored by an ImmunoCAP® system during SIT. Blood sampling was done before SIT (initial sampling, i) and 31.2±25.7 months after maintenance SIT (follow-up sampling, f). We performed an enzyme-linked immunosorbant assay (ELISA) to detect the changes of serum sIgE to alder, birch, ragweed and mugwort pollen extracts. Pollen extracts were prepared the same as described in a previous study method (17); pollen of Alnus incana (alder), Betula pendula (birch), Ambrosia artemisiifolia (ragweed), and Artemisia vulgaris (mugwort) were purchased at Allergon® (ThermoFisher Scientific, Uppsala, Sweden). After the preparation of a crude extraction in a phosphate buffered saline (PBS), a Bradford protein assay measured the protein concentration and a sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) confirmed the protein bands. ELISA was performed in sera sampled from two time-point with each pollen extract in a concentration of 5 µg/mL for 2 hr to detect the serum sIgE to each pollen. Serum sIgE levels were presented as absorbance values and positive cutoff levels were defined as (mean±3*standard deviation) of absorbance values from non-atopic healthy controls. IgE immunoblot analysis was performed with transfer 40 µg/mL pollen extracts to polyvinylidene difluoride (PVDF membrane, Millipore Co., Bedford, MA, USA) and incubation of sera for 12 hr at 4℃. The major allergen of each pollen was defined according to previous reports; Aln g 1 (17 kDa) in alder, Bet v 1 (17 kDa) in birch, Amb a 1 (38 kDa) in ragweed and Art v 1 (24 kDa) in mugwort (17, 18). A densitometry (Bio-Rad Universal Hood II Gel Imager, BIO-RAD Laboratories, Milan, Italy) observed the changes of IgE binding components.
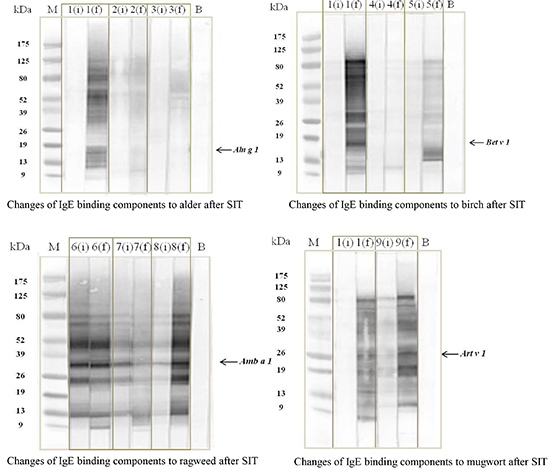
This study enrolled six male and three female pollinosis patients. Their mean age was 38.9±10.6 yr. All nine patients had seasonal AR and AC polysensitized to tree and weed pollens. BA was comorbid in five patients while two patients had AD. Initial sampling (i) was done before SIT and follow-up sampling (f) was done during SIT (31.2±25.7 months after maintenance SIT). All nine patients were treated with pollen-SIT with causative pollens that included the four pollens. Table 1 shows patient characteristics such as SPTs and ImmunoCAP® results. A Wilcoxon signed rank test compared the changes of serum sIgE levels in ELISA. Significant increases of serum sIgE levels to alder, birch, ragweed and mugwort pollen extracts by ELISA were noted during the maintenance of SIT compared to the initial levels presented in Fig. 1 (alder, P=0.001; birch, P=0.006; ragweed, P=0.001; mugwort, P=0.001). IgE immunoblot analysis showed two changing patterns: 1) intensification or a new generation of major allergen and 2) generation of additional IgE binding components (Fig. 2). Fig. 2A shows the changing patterns of alder pollen extracts; two patients (No. 1, 2) showed a new generation of major allergen (17 kDa) and additional IgE binding components (9-175 kDa); however, one patient (No. 3) showed a new generation of additional components (39-125 kDa, Fig. 2A). Fig. 2B showed the changing patterns of birch pollens. Two patients (No. 1, 5) showed new generations of major allergen (17 kDa) and additional components (9-125 kDa) while one patient (No. 4) showed new generation of additional components (11 kDa). Fig. 2C showed changing patterns of ragweed pollens; various changes of major allergen (38 kDa, showed no change in patient No. 6, decreased in No. 7, and intensified in No. 8) and a new generation of additional components ranged from 9-13 kDa. Fig. 2D showed the patterns of mugwort pollens; intensification (No. 9) or new generation (No. 1) of major (24 kDa) and additional components (9-80 kDa) were noted (Fig. 2D).
Serum sIgE levels increased at the first year of SIT and then decreased along with an increase of a blocking antibody, IgG and immunomodulation of B-cell itself or by regulatory T-cells (6, 19). We demonstrated increased sIgE levels to major tree and weed pollens (that included alder, birch, ragweed and mugwort pollens) in a subpopulation of pollinosis patients despite a few years of maintenance SIT with corresponding allergen extracts. ELISA confirmed increased serum sIgE levels and an IgE immunoblot analysis confirmed the intensification of major allergens or a new generation of IgE binding components to each pollen. Recent studies have demonstrated that environmental changes (especially climate change) can increase the allergenic potency of major allergens in pollens such as birch, ragweed and Japanese hop (10, 11, 12, 16). Our previous study demonstrated that environmental changes over the last 10 yr could induce the intensification of major allergenic component of Japanese hop pollen at 10 kDa and a new generation of additional IgE binding components within the pollens. The findings suggest that climate change can increase the allergenic potency of major pollens through intensification or the generation of new IgE binding components within major tree or weed pollens (alder, birch, ragweed and mugwort pollens) that can contribute to the increased prevalence and severity of pollinosis in Korea (10, 11, 12, 13, 14, 15, 20). The present study demonstrates that sIgE levels to offending allergens did not decrease; conversely, the results indicated a paradoxical increase in IgE binding components to alder, birch, ragweed and mugwort pollens after allergen-SIT in a subpopulation of pollinosis patients that could reduce clinical outcomes of SIT. The results suggest that the regular monitoring of serum sIgE levels and symptom changes in patients undergoing SIT with tree and weed pollens is needed to detect and improve clinical outcomes of SIT in patients who may have a paradoxical increase of sIgE.
Figures and Tables
Fig. 1
Changes of serum sIgE levels to tree and weed pollen extracts by ELISA. A Wilcoxon signed rank test indicates significant increase of sIgE levels to alder (A, from 1,110 ±1,234 to 1,938±1,065, P = 0.001), birch (B, from 1,201±1,495 to 1,994±1,290, P = 0.006), ragweed (C, from 401±677 to 1,490±986, P = 0.001) and mugwort (D, from 1,281±1,309 to 2,052±1,186, P = 0.001). Dashed line indicates cutoff values (172.7 in alder, 199.6 in birch, 186.2 in ragweed and 223.4 in mugwort). sIgE, specific IgE; ELISA, enzyme-linked immunosorbant assay.

Fig. 2
Changes of IgE binding components to tree and weed pollen extracts by immunoblotting. (A) In alder pollen, two patients (No. 1, 2) show new generations of IgE binding components to major allergen and additional components, and one (No. 3) shows a new generation of additional components. (B) In birch pollen, two patients (No. 1, 5) show a new generation of IgE binding components to major allergen and additional components; however, one patient (No. 4) shows a new generation of additional components. (C) Various changes of IgE binding component to major allergen (not changed in patient No. 6, decreased in No. 7, and intensified in No. 8) are noted in ragweed pollen along with a new generation of additional components that ranged from 9-13 kDa. (D) In mugwort pollen, a new generation of IgE binding components to both of major allergen and additional components (No. 1) and intensification of IgE binding components to major and additional components (No. 9) are noted. Major band was defined as below; Aln g 1 (17 kDa) in alder, Bet v 1 (17 kDa) in birch, Amb a 1 (38 kDa) in ragweed and Art v 1 (24 kDa) in mugwort.
Notes
References
1. Oh JW, Lee HB, Kang IJ, Kim SW, Park KS, Kook MH, Kim BS, Baek HS, Kim JH, Kim JK, et al. The revised edition of Korean calendar for allergenic pollens. Allergy Asthma Immunol Res. 2012; 4:5–11.
2. Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010; 2:65–76.
3. Jung JW, Choi JC, Shin JW, Kim JY, Park IW, Choi BW. Clinical characteristics according to sensitized allergens in adult Korean patients with bronchial asthma. Allergy Asthma Immunol Res. 2010; 2:102–107.
4. Zhang Y, Zhang L. Prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. 2014; 6:105–113.
5. Lee JM. Practice patterns of allergen immunotherapy in Korea: where are we? Allergy Asthma Immunol Res. 2013; 5:249–250.
6. Lee JE, Ahn JC, Han DH, Kim DY, Kim JW, Cho SH, Park HW, Rhee CS. Variability of offending allergens of allergic rhinitis according to age: optimization of skin prick test allergens. Allergy Asthma Immunol Res. 2014; 6:47–54.
7. Dretzke J, Meadows A, Novielli N, Huissoon A, Fry-Smith A, Meads C. Subcutaneous and sublingual immunotherapy for seasonal allergic rhinitis: a systematic review and indirect comparison. J Allergy Clin Immunol. 2013; 131:1361–1366.
8. Hur GY, Kim TB, Han MY, Nahm DH, Park JW. Allergen and Immunotherapy Work Group of the Korean Academy of Asthma, Allergy and Clinical Immunology (KAAACI). A survey of the prescription patterns of allergen immunotherapy in Korea. Allergy Asthma Immunol Res. 2013; 5:277–282.
9. Matsuoka T, Shamji MH, Durham SR. Allergen immunotherapy and tolerance. Allergol Int. 2013; 62:403–413.
10. Ziska LH, Beggs PJ. Anthropogenic climate change and allergen exposure: the role of plant biology. J Allergy Clin Immunol. 2012; 129:27–32.
11. D'Amato G, Rottem M, Dahl R, Blaiss M, Ridolo E, Cecchi L, Rosario N, Motala C, Ansotegui I, Annesi-Maesano I. Climate change, migration, and allergic respiratory diseases: an update for the allergist. World Allergy Organ J. 2011; 4:120–125.
12. Jin HJ, Choi GS, Shin YS, Kim JH, Kim JE, Ye YM, Park HS. The allergenic potency of Japanese hop pollen is increasing with environmental changes in Korea. Allergy Asthma Immunol Res. 2013; 5:309–314.
13. Dapul-Hidalgo G, Bielory L. Climate change and allergic diseases. Ann Allergy Asthma Immunol. 2012; 109:166–172.
14. Weber RW. Impact of climate change on aeroallergens. Ann Allergy Asthma Immunol. 2012; 108:294–299.
15. Lin GC, Zacharek MA. Climate change and its impact on allergic rhinitis and other allergic respiratory diseases. Curr Opin Otolaryngol Head Neck Surg. 2012; 20:188–193.
16. Kim JH, Oh JW, Lee HB, Kim SW, Kang IJ, Kook MH, Kim BS, Park KS, Baek HS, Kim KR, et al. Changes in sensitization rate to weed allergens in children with increased weeds pollen counts in Seoul Metropolitan area. J Korean Med Sci. 2012; 27:350–355.
17. Yoon MG, Kim MA, Jin HJ, Shin YS, Park HS. Identification of immunoglobulin E binding components of two major tree pollens, birch and alder. Allergy Asthma Respir Dis. 2013; 1:216–220.
18. Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy. 2010; 40:1442–1460.
19. Shamji MH, Ljørring C, Würtzen PA. Predictive biomarkers of clinical efficacy of allergen-specific immunotherapy: how to proceed. Immunotherapy. 2013; 5:203–206.
20. Kim J, Lee SW, Woo SY, Han YS, Lee JH, Lee IY, Lim IS, Choi ES, Choi BW, Cheong HK, et al. The indoor level of house dust mite allergen is associated with severity of atopic dermatitis in children. J Korean Med Sci. 2013; 28:74–79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download